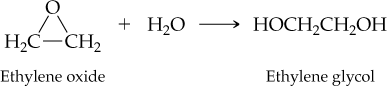Back
BackProblem 6
Which weighs more, 5.00 g or 0.0225 mol of acetaminophen (C8H9O2)?
Problem 8a
Balance the following equation, and tell how many moles of nickel will react with 9.81 mol of hydrochloric acid.
Ni(s) + HCl(aq) → NiCl2(aq) + H2(g)
Problem 8b
How many moles of NiCl2 can be formed in the reaction of 6.00 mol of Ni and 12.0 mol of HCl?
Ni(s) + HCl(aq) → NiCl2(aq) + H2(g)
Problem 13
The reaction of ethylene oxide with water to give ethylene glycol (automobile antifreeze) occurs in 96.0% actual yield. How many grams of ethylene glycol are formed by reaction of 35.0 g of ethylene oxide? (For ethylene oxide, MW = 44.0 amu; for ethylene glycol, MW = 62.0 amu.)
Problem 16b
The following diagram represents the reaction of A2(red spheres) with B2(blue spheres):
<IMAGE>
b. How many moles of product can be made from 1.0 mol of A2? From 1.0 mol of B2?
Problem 17
Consider the balanced chemical equation: 2A + B2 → 2AB. Given the following reaction vessel, determine the theoretical yield of product.
<IMAGE>
Problem 18
Consider the balanced chemical equation: A2 + 2 B2 → 2 AB2. A reaction is performed with the initial amounts of A2 and B2 shown in part (a). The amount of product obtained is shown in part (b). Calculate the percent yield.
a. <IMAGE>
b. <IMAGE>
Problem 22
How many Na+ ions are in a mole of Na2SO4? How many SO42- ions?
Problem 23
How many moles of ions are in 1.75 mol of K2SO4?
Problem 29
Caffeine has the formula C8H10N4O2. If an average cup of coffee contains approximately 125 mg of caffeine, how many moles of caffeine are in one cup?
Problem 35
The principal component of many kidney stones is calcium oxalate, CaC2O4. A kidney stone recovered from a typical patient contains 8.5 × 1020 formula units of calcium oxalate. How many moles of CaC2O4 are present in this kidney stone? What is the mass of the kidney stone in grams?
Problem 43a
Titanium metal is obtained from the mineral rutile, TiO2. The process requires multiple steps, as shown in the following reactions:
TiO2(s) + 2 Cl2(g) + 2 C(s) → TiCl4(s) + 2 CO(g)
TiCl4(s) + 2 Mg(s) → Ti(s) + 2 MgCl2(s)
a. Write mole ratios to show the relationship between the reactants and products for each reaction.
Problem 43b
Titanium metal is obtained from the mineral rutile, TiO2. The process requires multiple steps, as shown in the following reactions:
TiO2(s) + 2 Cl2(g) + 2 C(s) → TiCl4(s) + 2 CO(g)
TiCl4(s) + 2 Mg(s) → Ti(s) + 2 MgCl2(s)
b. How many moles of TiO2 are needed to form one mole of titanium?
Problem 43c
Titanium metal is obtained from the mineral rutile, TiO2. The process requires multiple steps, as shown in the following reactions:
TiO2(s) + 2 Cl2(g) + 2 C(s) → TiCl4(s) + 2 CO(g)
TiCl4(s) + 2 Mg(s) → Ti(s) + 2 MgCl2(s)
c. How many kilograms of rutile are needed to produce 95 kg of Ti?
Problem 50a
In Problem 6.40, hydrazine reacted with oxygen according to the following (unbalanced) equation: N2H4(l) + O2(g) → NO2(g) + H2O(g)
a. If 75.0 kg of hydrazine are reacted with 75.0 kg of oxygen, which is the limiting reagent?
Problem 53a
Nitrobenzene (C6H5NO2) is used in small quantities as a flavoring agent or in perfumes but can be toxic in large amounts. It is produced by reaction of benzene (C6H6) with nitric acid:
C6H6(l) + HNO3(aq) → C6H5NO2(l) + H2O(l)
a. Identify the limiting reagent in the reaction of 27.5 g of nitric acid with 75 g of benzene.
Problem 53b
Nitrobenzene (C6H5NO2) is used in small quantities as a flavoring agent or in perfumes but can be toxic in large amounts. It is produced by reaction of benzene (C6H6) with nitric acid:
C6H6(l) + HNO3(aq) → C6H5NO2(l) + H2O(l)
b. Calculate the theoretical yield for this reaction.
Problem 57a
When table sugar (sucrose, C12H22O11) is heated, it decomposes to form C and H2O.
a. Write a balanced equation for the process.
Problem 57b
When table sugar (sucrose, C12H22O11) is heated, it decomposes to form C and H2O.
b. How many grams of carbon are formed by the breakdown of 60.0 g of sucrose?
Problem 58b
Although Cu is not sufficiently active to react with acids, it can be dissolved by concentrated nitric acid, which functions as an oxidizing agent according to the following equation:
Cu(s) + 4 HNO3(aq) → Cu(NO3)2(aq) + 2 NO2(g) + 2 H2O(l)
b. Is 35.0 g of HNO3 sufficient to dissolve 5.00 g of copper?
Problem 60
Ethyl alcohol is formed by enzyme action on sugars and starches during fermentation.
C6H12O6 → 2 CO2 + 2 C2H6O
If the density of ethyl alcohol is 0.789 g/mL, how many quarts can be produced by the fermentation of 100.0 lb of sugar?
Problem 65a
Acetylsalicylic acid, the active ingredient in aspirin, is prepared from salicylic acid by reaction with acetic anhydride.
C7H6O3 (salicylic acid) + C4H6O3 (acetic anhydride) → C9H8O4 (acetylsalicylic acid) + C2H4O2 (acetic acid)
a. Calculate the theoretical yield if 47 g of salicylic acid is reacted with 25 g of acetic anhydride.
Problem 67a
Elemental phosphorus exists as molecules of P4. It reacts with Cl2(g) to produce phosphorus pentachloride.
a. Write the balanced chemical equation for this reaction.
Problem 70a
Calcium citrate, Ca(C6H5O7)2(MW = 498.5 amu), is a common dietary supplement to provide calcium needed for strong teeth and bones.
a. Look up the recommended daily dietary intake of calcium for adult men and premenopausal women.
Problem 70b
Calcium citrate, Ca(C6H5O7)2(MW = 498.5 amu), is a common dietary supplement to provide calcium needed for strong teeth and bones.
b. What mass of calcium citrate would be needed to provide the recommended daily intake of calcium?
Problem 71a
Obtain a bottle of aspirin and identify the amount of active ingredient (acetylsalicylic acid, C9H8O4) per tablet.
a. How many moles of aspirin are in one tablet?
Problem 72a
Lovastatin, a drug used to lower serum cholesterol.
a. Look up the molecular formula for Lovastatin and calculate the molar mass.
Problem 73b
Pyrite, also known as fool's gold, is used commercially to produce SO2 used in the production of paper products.
b. How many moles of SO2 can be produced from 1.0 kg of pyrite?