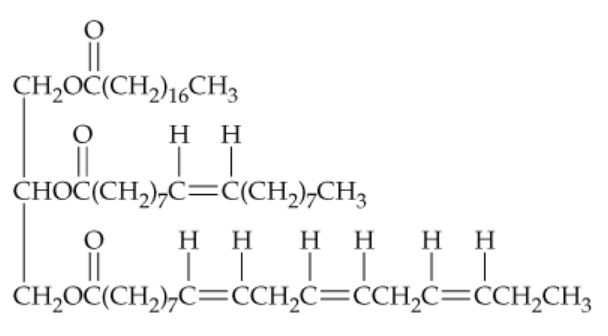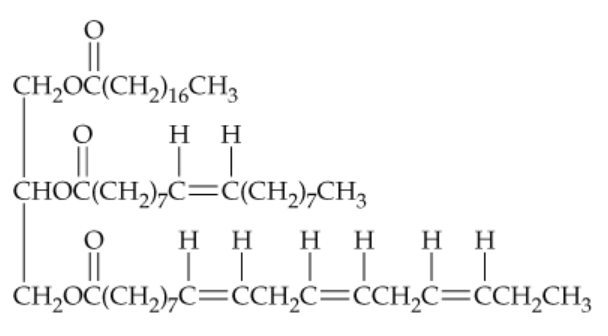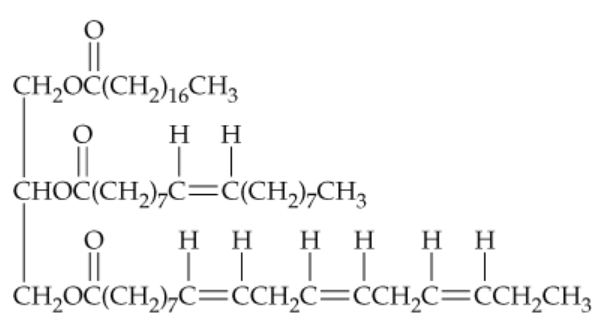Back
BackProblem 1b
Use Figure 23.1 to identify the family of lipids to which each of these molecules belongs.
b.
Problem 2
One of the constituents of the carnauba wax used in floor and furniture polish is an ester of a 32-carbon straight-chain alcohol with a C20:0 straight-chain carboxylic acid. Draw the structure of this ester. (Use subscripts to show the numbers of connected CH2 groups.)
Problem 5
Draw the complete structural formula of arachidonic acid (Table 23.1) in a way that shows the cis stereochemistry of its four double bonds.
Problem 6
Can there be any chiral carbon atoms in triacylglycerols? If so, which ones can be chiral and what determines their chirality?
Problem 8
Write an equation for the complete hydrogenation of triolein, the triacylglycerol with three oleic acid acyl groups for which you drew the structure in Problem 23.3. Name the fatty acid from which the resulting acyl groups are derived.
Problem 11
Write the complete equation for the hydrolysis of a triacylglycerol in which the fatty acids are two molecules of stearic acid and one of oleic acid.
Problem 13a
Identify the products formed by complete hydrolysis of all ester bonds in (a) the phosphatidylcholine on page 726.
Problem 13b
Identify the products formed by complete hydrolysis of all ester bonds in (b) the sphingomyelin in Figure 23.5.
Problem 14
Draw the structure of the sphingomyelin that contains a myristic acid acyl group. Identify the hydrophilic head group and the hydrophobic tails in this molecule.
Problem 15
Draw the structure of the glycerophospholipid that contains a stearic acid acyl group, an oleic acid acyl group, and a phosphate bonded to ethanolamine.
Problem 16a
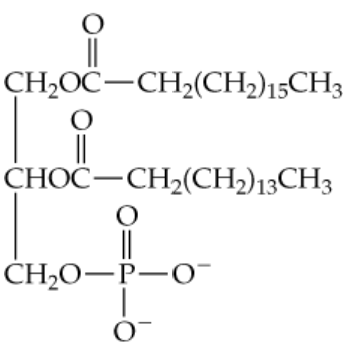
Which of the following terms apply to the compound shown below? (Hint: Look at the functional groups and the bonds involved to begin analyzing the compound part by part in comparison to the lipids discussed in this chapter.)
a. A phospholipid
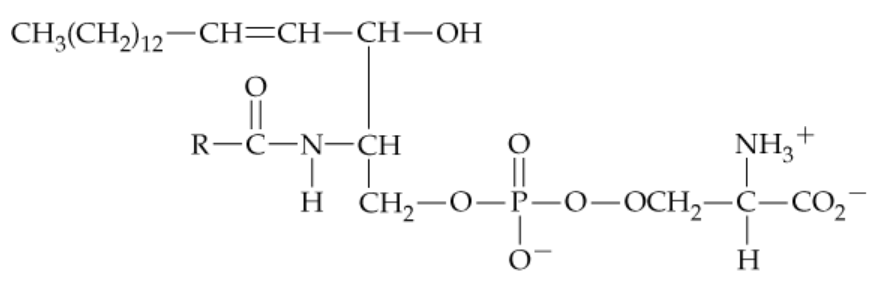
Problem 16e
Which of the following terms apply to the compound shown below? (Hint: Look at the functional groups and the bonds involved to begin analyzing the compound part by part in comparison to the lipids discussed in this chapter.)
e. A lipid
Problem 19
As noted earlier (Section 22.3), he first step in glycolysis, which occurs within cells, is phosphorylation of glucose to glucose 6-phosphate. Why does this step prevent passive diffusion of glucose back out of the cell?
Problem 22
Complete hydrogenation of triacylglycerol C in Problem 23.20 yields a triacylglycerol of what fatty acid composition? Would the hydrogenation product of triacylglycerol C be more like the hydrogenation product of triacylglycerol A or B? Explain.
Problem 23
A membrane lipid was isolated and completely hydrolyzed. The following products were detected: ethanolamine, phosphate, glycerol, palmitic acid, and oleic acid. Propose a structure for this membrane lipid, and name the family to which it belongs.
Problem 28
Draw an 18-carbon saturated fatty acid. Is this a “straight-chain” molecule or a “bent” molecule?
Problem 29
Draw an 18-carbon unsaturated fatty acid that contains two carbon–carbon double bonds, one on carbon 6 and one on carbon 9 (count starting with the carboxyl carbon). Is this a “straight-chain” molecule or a “bent” molecule?
Problem 31
Are the carbon–carbon double bonds in naturally occurring fatty acids primarily cis or trans?
Problem 34
Which of these fatty acids has the lower melting point? Explain why.
a. Linoleic acid
b. Stearic acid
Problem 35
Which of these fatty acids has the higher melting point? Explain why.
a. Linolenic acid
b. Stearic acid
Problem 38
Draw the structure of glyceryl trilaurate, which is made from glycerol and three lauric acid molecules.
Problem 39
There are two isomeric triacylglycerol molecules whose components are glycerol, one palmitic acid unit, and two stearic acid units. Draw the structures of both, and explain how they differ.
Problem 48
Is the reaction shown here esterification, hydrogenation, hydrolysis, saponification, or substitution?
Problem 49
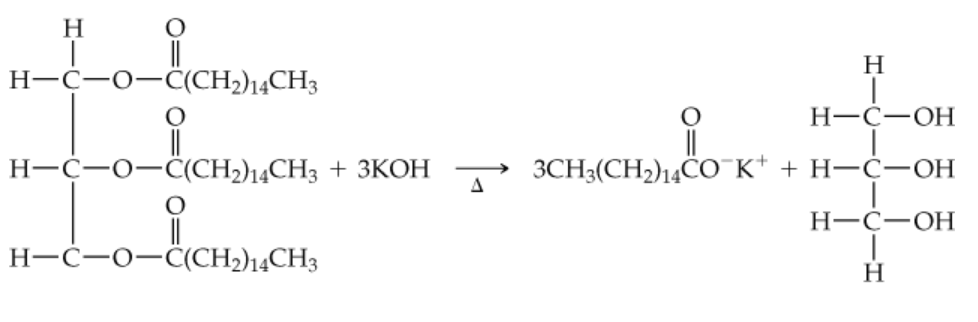
Draw the structures of all products you would obtain by saponification of the following lipid with aqueous KOH. What are the names of the products?
Problem 50
Draw the structure of the product you would obtain on complete hydrogenation of the triacylglycerol in Problem 23.49. What is its name? Does it have a higher or lower melting temperature than the original triacylglycerol?
Problem 51
Tell how many different products you would obtain on hydrogenation of the triacylglycerol in Problem 23.49 if:
a. One double bond was converted to a single bond
b. Two double bonds were converted to single bonds
c. Three double bonds were converted to single bonds
d. All four double bonds were converted to single bonds
Problem 52a
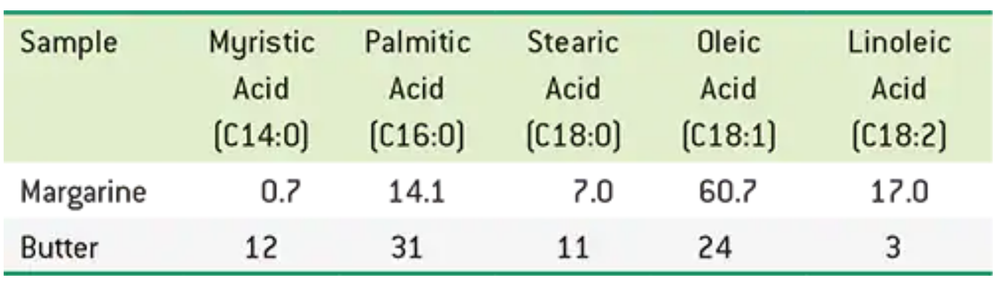
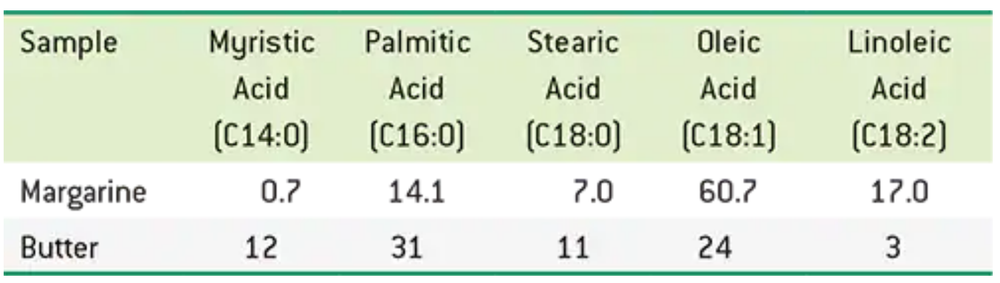
Dietary guidelines suggest we limit our intake of butter due to the cholesterol content and substitute oils or margarine. The following table shows the major fatty acid distribution for a typical stick of margarine and also for butter. Values are percentages.
a. Which contains more monounsaturated fatty acids?
Problem 52c
Dietary guidelines suggest we limit our intake of butter due to the cholesterol content and substitute oils or margarine. The following table shows the major fatty acid distribution for a typical stick of margarine and also for butter. Values are percentages.
c. Which is likely to contain fewer trans-fatty acids
Problem 58
Why are glycerophospholipids more soluble in water than triacylglycerols?
Problem 60
Show the structure of a cerebroside made up of D-galactose, sphingosine, and myristic acid.