Identify the type of reaction for the following:
a.
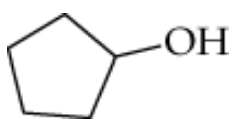
b.

 Verified step by step guidance
Verified step by step guidance
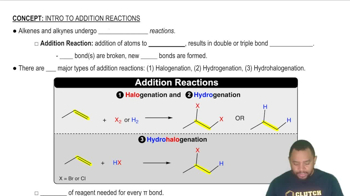

Identify the type of reaction for the following:
a.
b.
What alkene could you use to make the following products? Draw the structure of the alkene, and tell what other reagent is also required for the reaction to occur.
a.
What alkene could you use to make the following products? Draw the structure of the alkene, and tell what other reagent is also required for the reaction to occur.
c.
2,2,3,3-Tetrabromopentane can be prepared by an addition reaction of excess Br2 with an alkyne. Draw the structure of the alkyne and name it.
1-Pentyne reacts with HBr in a 1:1 molar ratio to yield two different addition products, both being bromopentenes and having the chemical formula C5H9Br. Draw the structures of two possible products.
Polyvinylpyrrolidone (PVP) is often used in hair sprays to hold hair in place. Draw a few units of the PVP polymer. The vinylpyrrolidone monomer unit has the following structure: