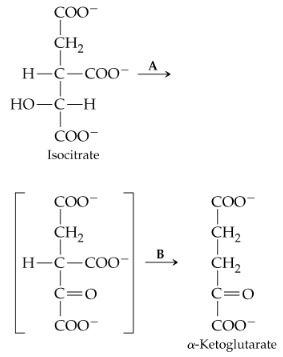
The reaction that follows is catalyzed by isocitrate dehydrogenase and occurs in two steps, the first of which (step A) is formation of an unstable intermediates (shown in brackets).
a. In which step is a coenzyme needed? Identify the coenzyme.

 Verified step by step guidance
Verified step by step guidance

The reaction that follows is catalyzed by isocitrate dehydrogenase and occurs in two steps, the first of which (step A) is formation of an unstable intermediates (shown in brackets).
a. In which step is a coenzyme needed? Identify the coenzyme.
The reaction that follows is catalyzed by isocitrate dehydrogenase and occurs in two steps, the first of which (step A) is formation of an unstable intermediates (shown in brackets).
b. In which step is CO2 evolved and a hydrogen ion added?
The reaction that follows is catalyzed by isocitrate dehydrogenase and occurs in two steps, the first of which (step A) is formation of an unstable intermediates (shown in brackets).
c. Which of the structures shown can be described as a β-keto acid?
The electron-transport chain uses several different metal ions, especially iron, copper, zinc, and manganese. Why are metals used frequently in these two pathways? What can metals do better than organic biomolecules?
What energy requirements must be met in order for a reaction to be favorable?
Why is ∆G a useful quantity for predicting the favorability of biochemical reactions?