Answer questions (a)–(e) concerning the following reaction:
c. What is the substrate for the reaction as written?

 Verified step by step guidance
Verified step by step guidance
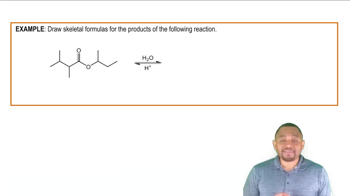
Answer questions (a)–(e) concerning the following reaction:
c. What is the substrate for the reaction as written?
Answer questions (a)–(e) concerning the following reaction:
d. What is the product for the reaction as written?
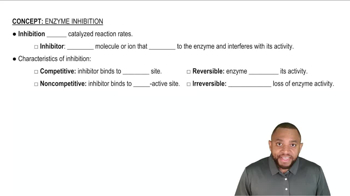
Explain how the following changes affect the rate of an enzyme-catalyzed reaction in the presence of an uncompetitive inhibitor:
(a) increasing the substrate concentration at a constant inhibitor concentration
Explain how the following mechanisms regulate enzyme activity.
b. Genetic control
What type of enzyme regulation occurs in the following situations?
a. Buildup of the product of the pathway that converts glucose to pyruvate stops at the first enzyme in the multistep process.
What type of enzyme regulation occurs in the following situations?
d. Conversion of isocitrate to α-ketoglutarate is inhibited by high levels of ATP. (Hint: ATP is neither a product nor a substrate in this reaction.)