Draw the structures of the following compounds and use dashed lines to indicate where they form hydrogen bonds to other molecules of the same kind: (ii) methyl formate
Ch.17 Carboxylic Acids and Their Derivatives

Chapter 17, Problem 34c
Consider the following unnatural amino acid:
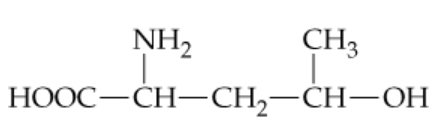
c. Draw the cyclic ester resulting from the intramolecular reaction of the hydroxyl group of this amino acid with its carboxyl group (cyclic esters are called lactones).
 Verified step by step guidance
Verified step by step guidance1
Identify the functional groups in the given unnatural amino acid: a hydroxyl (-OH) group and a carboxyl (-COOH) group. These groups are key to forming the cyclic ester (lactone).
Recognize that the intramolecular reaction involves the nucleophilic attack of the hydroxyl group (-OH) on the carbonyl carbon of the carboxyl group (-COOH). This reaction forms an ester bond (-COO-).
Determine the size of the ring that will form. Count the number of atoms in the chain between the hydroxyl group and the carboxyl group, including the oxygen atom from the hydroxyl group and the carbonyl carbon. This will help you predict whether the lactone is a 5-membered or 6-membered ring.
Draw the structure of the cyclic ester (lactone). Ensure that the hydroxyl group is now part of the ester bond, and the carboxyl group has been converted into the ester functional group. The ring should include the appropriate number of atoms based on the chain length.
Verify the structure by checking that all atoms have the correct number of bonds and that the cyclic ester is properly formed. Ensure that the reaction conserves the molecular formula of the original amino acid.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Cyclic Esters (Lactones)
Cyclic esters, known as lactones, are formed when a hydroxyl group reacts with a carboxyl group within the same molecule, resulting in a ring structure. This reaction typically occurs through a condensation process, where water is eliminated. Lactones are significant in organic chemistry and biochemistry, as they can influence the properties and reactivity of compounds, including amino acids.
Recommended video:
Guided course
Cyclic Hemiacetals Concept 2
Intramolecular Reactions
Intramolecular reactions occur within a single molecule, where functional groups react with each other to form new structures. In the context of amino acids, this can lead to the formation of cyclic compounds, such as lactones, which can affect the molecule's stability and biological activity. Understanding these reactions is crucial for predicting the behavior of amino acids in various chemical environments.
Recommended video:
Guided course

Intermolecular Forces (Simplified) Example 1
Amino Acid Structure
Amino acids are organic compounds characterized by a central carbon atom bonded to an amino group, a carboxyl group, a hydrogen atom, and a variable R group (side chain). The specific structure of the R group determines the properties and classification of the amino acid. In the case of unnatural amino acids, modifications to the standard structure can lead to unique reactivity and the potential for forming cyclic esters through intramolecular reactions.
Recommended video:
Guided course

Amino Acid Catabolism: Amino Group Example 2
Related Practice
Textbook Question
Textbook Question
N-Acetylglucosamine (also known as NAG) is an important component on the surfaces of cells.
b. Draw the structures of the products of acid hydrolysis.
Textbook Question
One phosphorylated form of glycerate is 3-phosphoglycerate
a. Identify the type of linkage between glycerate and phosphate.
Textbook Question
Consider the following unnatural amino acid:
a. If two molecules react to form an ester, what is the structure of the ester product?
Textbook Question
Draw the structures of the following compounds and use dashed lines to indicate where they form hydrogen bonds to other molecules of the same kind: (i) formic acid
Textbook Question
Arrange these compounds in order of increasing boiling points and explain your rationale for the order.
(i) formic acid
(ii) methyl formate
(iii) formamide.
