How do amines differ from analogous alcohols in (a) odor, (b) basicity, and (c) boiling point?
Ch.16 Amines

Chapter 16, Problem 53c
Complete the following equations (Hint: Answers may include concepts learned from previous organic chapters):
c. 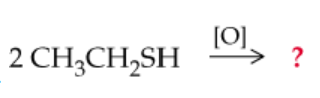
 Verified step by step guidance
Verified step by step guidance1
Identify the type of reaction taking place in the given equation. For example, determine if it is a substitution, addition, elimination, or oxidation-reduction reaction. This will guide how the reactants transform into products.
Analyze the functional groups present in the reactants. Functional groups such as alcohols, alkenes, alkynes, or carbonyl groups often dictate the type of reaction and the products formed.
Apply the appropriate reaction mechanism or rule. For example, if the reaction involves an alkene, consider Markovnikov's or anti-Markovnikov's rule for addition reactions, or if it involves an alcohol, consider oxidation or dehydration pathways.
Balance the chemical equation by ensuring that the number of atoms of each element is the same on both sides of the equation. Also, ensure that the charges are balanced if ions are involved.
Double-check the products to ensure they are consistent with the reaction type and the reagents used. Verify that the stereochemistry (if applicable) and connectivity of atoms in the products are correct.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Chemical Equations
Chemical equations represent the transformation of reactants into products during a chemical reaction. They provide a concise way to convey the quantities and types of substances involved, using symbols and formulas. Understanding how to balance these equations is crucial, as it reflects the law of conservation of mass, ensuring that the number of atoms remains constant throughout the reaction.
Recommended video:
Guided course
Balancing Chemical Equations (Simplified) Concept 1
Functional Groups
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. In organic chemistry, recognizing functional groups is essential for predicting the behavior of compounds during reactions. Examples include hydroxyl (-OH), carboxyl (-COOH), and amino (-NH2) groups, each imparting unique properties to the molecules they are part of.
Recommended video:
Guided course
Functional Group Priorities Concept 1
Reaction Mechanisms
Reaction mechanisms describe the step-by-step process by which reactants are converted into products in a chemical reaction. Understanding these mechanisms helps in predicting the outcomes of reactions and the conditions required for them to occur. Key concepts include intermediates, transition states, and the energy changes associated with each step, which are vital for grasping how and why reactions proceed in a particular manner.
Recommended video:
Guided course
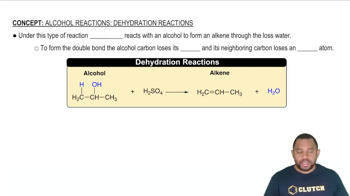
Alcohol Reactions: Dehydration Reactions Concept 1
Related Practice
Textbook Question
Textbook Question
Name the following compounds:
a.
Textbook Question
Complete the following equations (Hint: Answers may include concepts learned from previous organic chapters):
a.
Textbook Question
Complete the following equations (Hint: Answers may include concepts learned from previous organic chapters):
e.
Textbook Question
Baeocystin is a hallucinogenic compound that is isolated from the mushroom Psilocybe baeocystis and has the structure shown below. What heterocyclic base (Table 16.1) is the parent of this compound?
Textbook Question
Benzene and pyridine are both single-ring, aromatic compounds. Benzene is a neutral compound that is insoluble in water. Pyridine, with a similar molar mass, is basic and completely miscible with water. Explain these phenomena.
