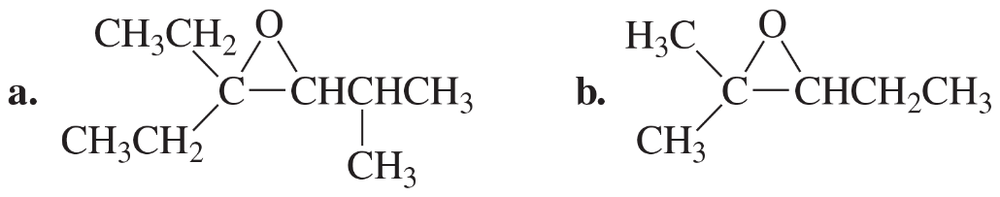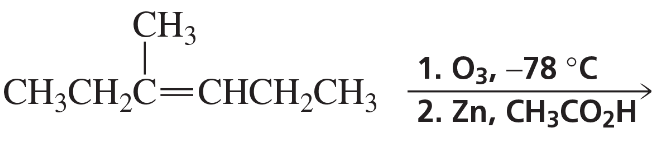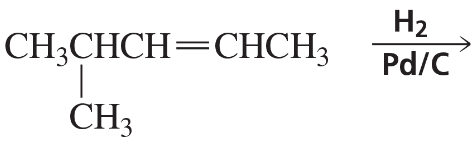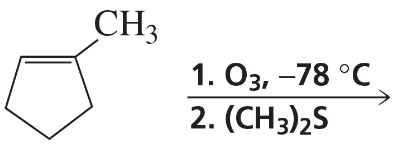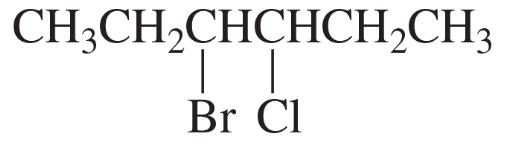Back
Back Bruice 8th Edition
Bruice 8th Edition Ch.6 - The Reactions of Alkenes The Stereochemistry of Addition Reactions
Ch.6 - The Reactions of Alkenes The Stereochemistry of Addition ReactionsProblem 57b
Identify the electrophile and the nucleophile in each of the following reaction steps and then draw curved arrows to illustrate the bond-making and bondbreaking processes.
b.
Problem 57c
Identify the electrophile and the nucleophile in each of the following reaction steps and then draw curved arrows to illustrate the bond-making and bondbreaking processes.
c.
Problem 58a
What is the major product of the reaction of 2-methyl-2-butene with each of the following reagents?
a. HBr
Problem 58b
What is the major product of the reaction of 2-methyl-2-butene with each of the following reagents?
b. HI
Problem 58d
What is the major product of the reaction of 2-methyl-2-butene with each of the following reagents?
d. O3, −78 °C, followed by (CH3)2S
Problem 58f
What is the major product of the reaction of 2-methyl-2-butene with each of the following reagents?
f. MCPBA (a peroxyacid)
Problem 58g
What is the major product of the reaction of 2-methyl-2-butene with each of the following reagents?
g. H2O + H2SO4
Problem 58j
What is the major product of the reaction of 2-methyl-2-butene with each of the following reagents?
j. Br2/CH3OH
Problem 58k
What is the major product of the reaction of 2-methyl-2-butene with each of the following reagents?
k. BH3/THF, followed by H2O2, HO- , H2O
Problem 59
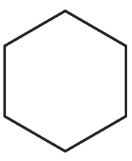
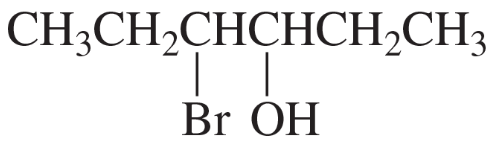
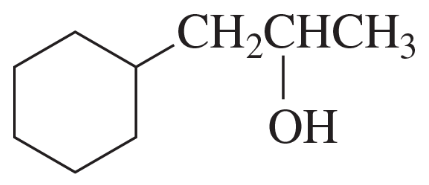
Give two names for each of the following:
Problem 60
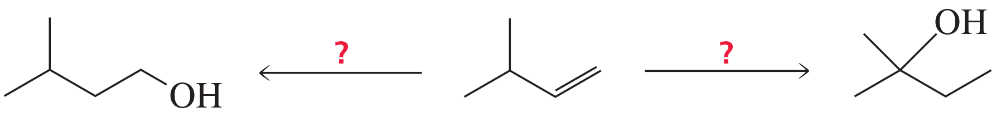
What reagents are needed to synthesize the following alcohols?
Problem 61a
What are the products of the following reactions? Indicate whether each reaction is an oxidation or a reduction.
a.
Problem 61b
What are the products of the following reactions? Indicate whether each reaction is an oxidation or a reduction.
b.
Problem 61c
What are the products of the following reactions? Indicate whether each reaction is an oxidation or a reduction.
c.
Problem 62a
When 3-methyl-1-butene reacts with HBr, two alkyl halides are formed: 2-bromo-3-methylbutane and 2-bromo-2-methylbutane. Propose a mechanism that explains the formation of these two products.
Problem 64a
What reagents are needed to carry out the following syntheses?
Problem 64b
What reagents are needed to carry out the following syntheses?
Problem 64c
What reagents are needed to carry out the following syntheses?
Problem 64d
What reagents are needed to carry out the following syntheses?
Problem 65
a. Identify two alkenes that react with HBr to form 1-bromo-1-methylcyclohexane without undergoing a carbocation rearrangement.
b. Would both alkenes form the same alkyl halide if DBr were used instead of HBr? (D is an isotope of H, so D+ reacts like H+.)
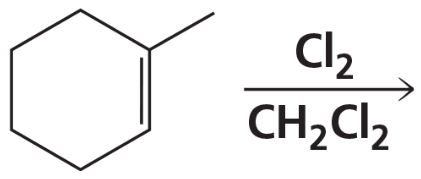
Problem 66a
What is the major product of each of the following reactions?
a.
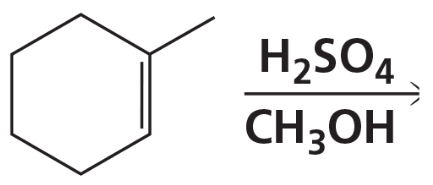
Problem 66b
What is the major product of each of the following reactions?
b.
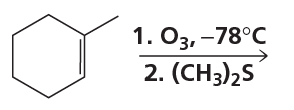
Problem 66e
What is the major product of each of the following reactions?
e.
Problem 66f
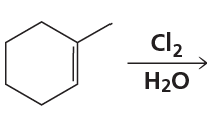
What is the major product of each of the following reactions?
f.
Problem 66g
What is the major product of each of the following reactions?
g.
Problem 66h
What is the major product of each of the following reactions?
h.
Problem 67a
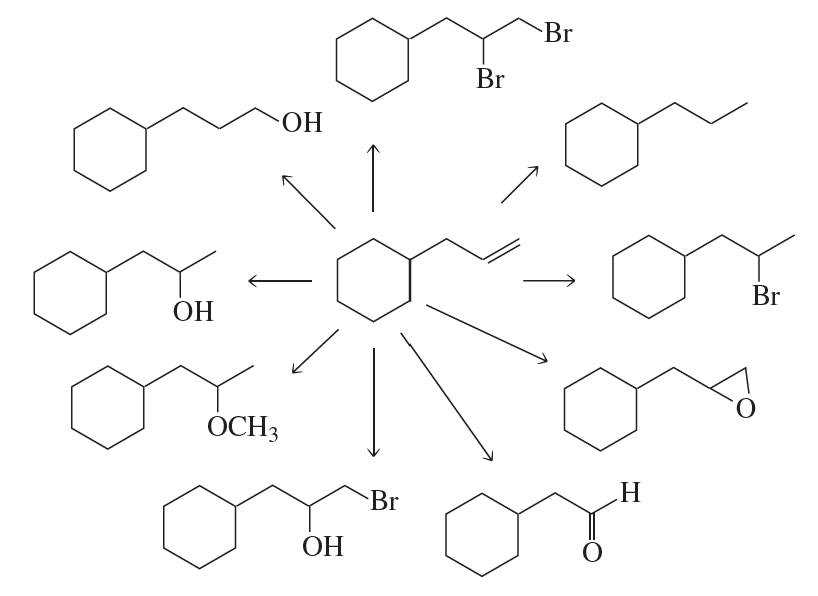
Using any alkene and any other reagents, how would you prepare the following compounds?
a.
Problem 67d
Using any alkene and any other reagents, how would you prepare the following compounds?
d.
Problem 67e
Using any alkene and any other reagents, how would you prepare the following compounds?
e.
Problem 67f
Using any alkene and any other reagents, how would you prepare the following compounds?
f.