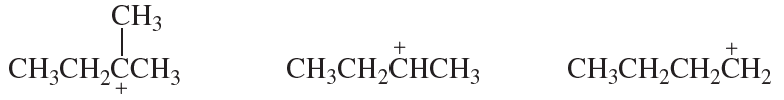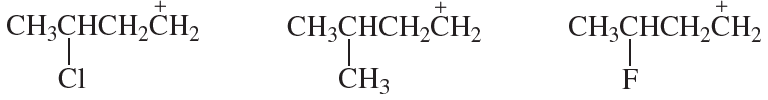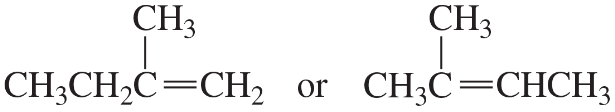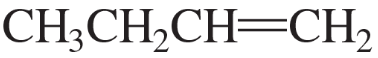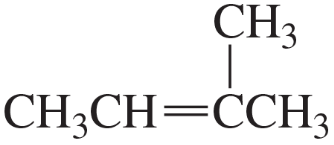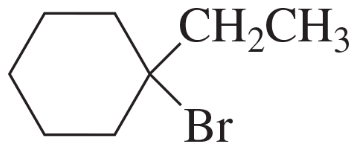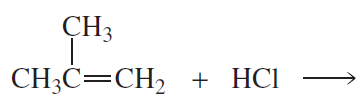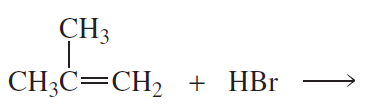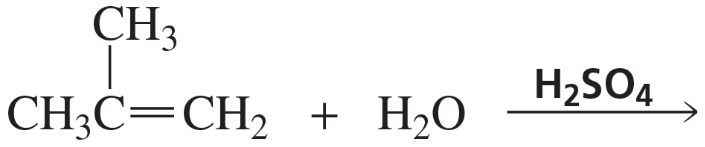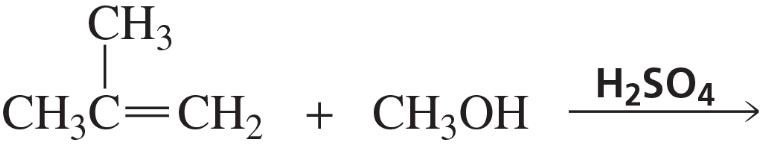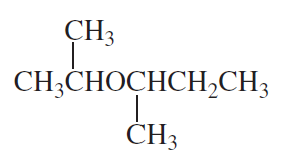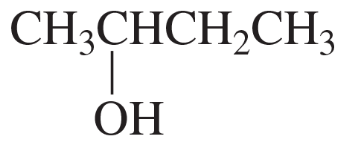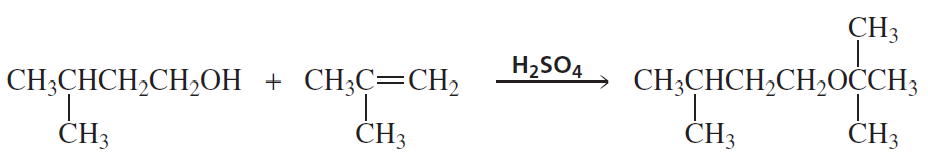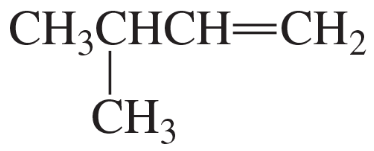Back
Back Bruice 8th Edition
Bruice 8th Edition Ch.6 - The Reactions of Alkenes The Stereochemistry of Addition Reactions
Ch.6 - The Reactions of Alkenes The Stereochemistry of Addition ReactionsProblem 1
Draw the mechanism for the reaction of cyclohexene with HCl
Problem 2a
How many s bond orbitals are available for overlap with the vacant p orbital in the methyl cation?
Problem 2b
Which is more stable: a methyl cation or an ethyl cation? Why?
Problem 3a
How many s bond orbitals are available for overlap with the vacant p orbital in
1. the isobutyl cation?
2. the n-butyl cation?
3. the sec-butyl cation?
Problem 3b
Which of the carbocations in part a is most stable?
1. the isobutyl cation?
2. the n-butyl cation?
3. the sec-butyl cation?
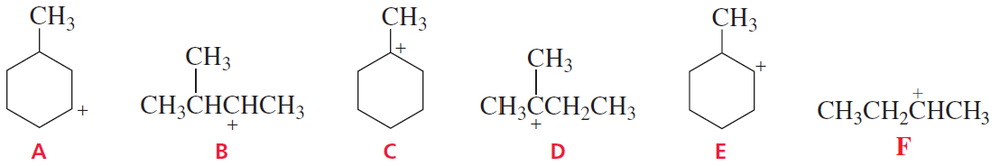
Problem 4a
Rank the following carbocations in each set from most stable to least stable:
a.
Problem 4b
Rank the following carbocations in each set from most stable to least stable:
b.
Problem 5
Is the structure of the transition state in the following reaction coordinate diagrams more similar to the structure of the reactant or to the structure of the product?
<IMAGE>
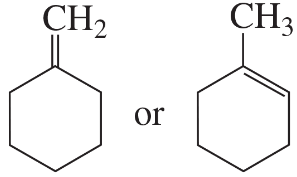
Problem 6a,b
To which compound is the addition of HBr more highly regioselective?
a.
b.
Problem 7a,b
What is the major product obtained from the addition of HBr to each of the following compounds?
a.
b.
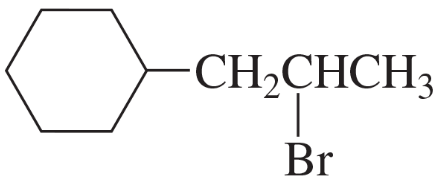
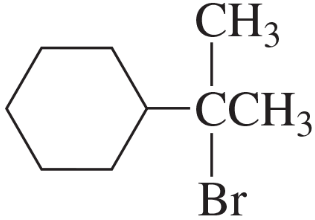
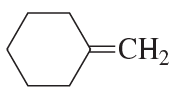
Problem 7e,f
What is the major product obtained from the addition of HBr to each of the following compounds?
e.
f.
Problem 8b
What alkene should be used to synthesize each of the following alkyl bromides?
b.
Problem 8c
What alkene should be used to synthesize each of the following alkyl bromides?
c.
Problem 8d
What alkene should be used to synthesize each of the following alkyl bromides?
d.
Problem 9a
The pKa of a protonated alcohol is about -2.5, and the pKa of an alcohol is about 15. Therefore, as long as the pH of the solution is greater than _______ and less than _______, more than 50% of 2-propanol (the product of the reaction on p. 244) will be in its neutral, nonprotonated form.
Problem 10a,b,c
Answer the following questions about the mechanism for the acid-catalyzed hydration of an alkene:
a. How many transition states are there?
b. How many intermediates are there?
c. Which step in the forward direction has the smallest rate constant?
Problem 11a
What is the major product obtained from the acid-catalyzed hydration of each of the following alkenes?
a. CH3CH2CH2CH=CH2
Problem 11d
What is the major product obtained from the acid-catalyzed hydration of each of the following alkenes?
d.
Problem 12a(1)
What is the major product of each of the following reactions?
1.
Problem 12a(2)
What is the major product of each of the following reactions?
2.
Problem 12a(3,4)
What is the major product of each of the following reactions?
3.
4.
Problem 13
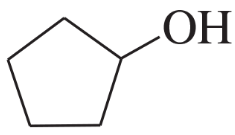
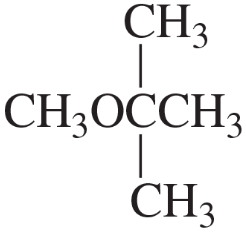
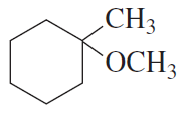
How could the following compound be prepared using an alkene as one of the starting materials?
Problem 14a
How could the following compounds be prepared using an alkene as one of the starting materials?
a.
Problem 14b
How could the following compounds be prepared using an alkene as one of the starting materials?
b.
Problem 14c
How could the following compounds be prepared using an alkene as one of the starting materials?
c.
Problem 14d
How could the following compounds be prepared using an alkene as one of the starting materials?
d.
Problem 14e
How could the following compounds be prepared using an alkene as one of the starting materials?
e.
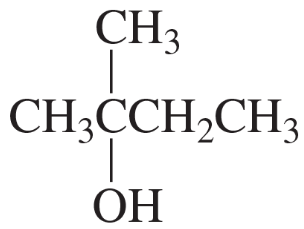
Problem 15
Propose a mechanism for the following reaction (remember to use curved arrows to show the movement of electrons from the nucleophile to the electrophile):
Problem 16a
Which of the following carbocations would you expect to rearrange?
Problem 17a
What is the major product obtained from the reaction of HBr with each of the following?
a.