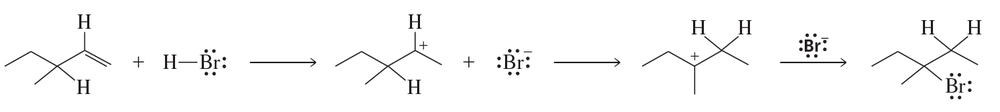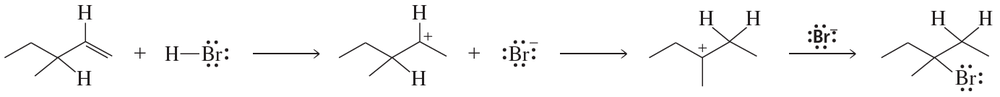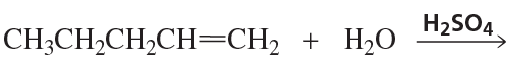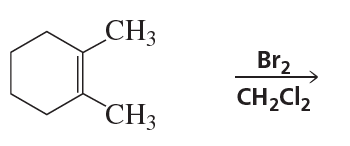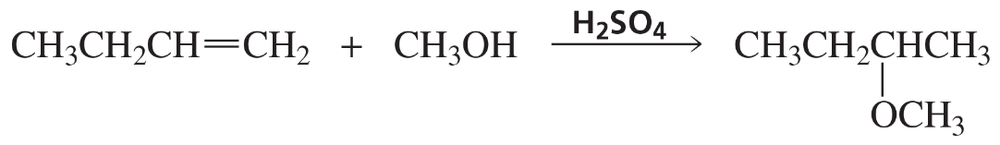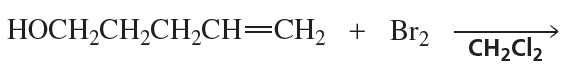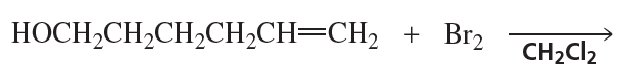Back
Back Bruice 8th Edition
Bruice 8th Edition Ch.6 - The Reactions of Alkenes The Stereochemistry of Addition Reactions
Ch.6 - The Reactions of Alkenes The Stereochemistry of Addition ReactionsProblem 69a
Use curved arrows to show the flow of electrons that occurs in each step of the following mechanism
Problem 69b
Draw a reaction coordinate diagram for the reaction. (Hint: An alkyl halide is more stable than an alkene.)
Problem 70(5)
a. Draw the product or products that will be obtained from the reaction of cis-2-butene and trans-2-butene with each of the following reagents. If a product can exist as stereoisomers, show which stereoisomers are formed.
5. Br2 + H2O
b. With which reagents do the two alkenes react to form different products?
Problem 70a(3)
Draw the product or products that will be obtained from the reaction of cis-2-butene and trans-2-butene with each of the following reagents. If a product can exist as stereoisomers, show which stereoisomers are formed.
3. a peroxyacid
Problem 70a,b(7)
a. Draw the product or products that will be obtained from the reaction of cis-2-butene and trans-2-butene with each of the following reagents. If a product can exist as stereoisomers, show which stereoisomers are formed.
7. H2O + H2SO4
b. With which reagents do the two alkenes react to form different products?
Problem 70a(2)
Draw the product or products that will be obtained from the reaction of cis-2-butene and trans-2-butene with each of the following reagents. If a product can exist as stereoisomers, show which stereoisomers are formed.
2. BH3/THF, followed by HO-, H2O2, H2O
Problem 70a(1)
Draw the product or products that will be obtained from the reaction of cis-2-butene and trans-2-butene with each of the following reagents. If a product can exist as stereoisomers, show which stereoisomers are formed.
1. HCl
Problem 70a(4)
Draw the product or products that will be obtained from the reaction of cis-2-butene and trans-2-butene with each of the following reagents. If a product can exist as stereoisomers, show which stereoisomers are formed.
4. Br2 in CH2Cl2
Problem 71
1-Methylcyclohexene forms two products when it reacts with bromine in methanol.
a. Draw the mechanism for the formation of the products.
b. Describe their stereochemical relationship—that is, are they enantiomers or diastereomers?
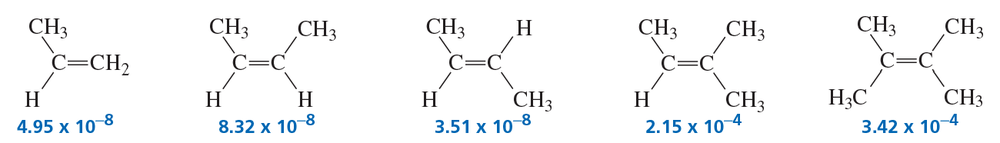
Problem 72
The second-order rate constant (in units of M-1s-1) for acid-catalyzed hydration at 25 °C is given for each of the following alkenes:
a. Calculate the relative rates of hydration of the alkenes. (Hint: Divide each rate constant by the smallest rate constant of the series: 3.51 × 10-8.)
b. Why does (Z)-2-butene react faster than (E)-2-butene?
c. Why does 2-methyl-2-butene react faster than (Z)-2-butene?
d. Why does 2,3-dimethyl-2-butene react faster than 2-methyl-2-butene?
Problem 74b
Draw the products of the following reactions. If the products can exist as stereoisomers, show which stereoisomers are formed.
b.
Problem 74c
Draw the products of the following reactions. If the products can exist as stereoisomers, show which stereoisomers are formed.
c.
Problem 75
A student was about to turn in the products he had obtained from the reaction of HI with 3,3,3-trifluoropropene when he realized that the labels had fallen off his flasks and he did not know which label belonged to which flask. His friend reminded him of the rule that says the electrophile adds to the sp2 carbon bonded to the most hydrogens. In other words, he should label the flask containing the most product 1,1,1-trifluoro-2-iodopropane and label the flask containing the least amount of product 1,1,1-trifluoro-3-iodopropane. Should he follow his friend’s advice?
Problem 76a
a. Propose a mechanism for the following reaction (show all curved arrows):
Problem 76b
b. Which step is the rate-determining step?
Problem 76c,d
c. What is the electrophile in the first step?
d. What is the nucleophile in the first step?
Problem 76e,f
e. What is the electrophile in the second step?
f. What is the nucleophile in the second step?
Problem 77b
Draw the products, including their configurations, obtained from the reaction of 1-ethylcyclohexene with the following reagents:
b. H2, Pd/C
Problem 77c
Draw the products, including their configurations, obtained from the reaction of 1-ethylcyclohexene with the following reagents:
c. R2BH/THF, followed by HO–, H2O2, H2O
Problem 78
Which stereoisomer of 3-hexene forms a meso compound when it reacts with Br2?
Problem 79
Which stereoisomer of 3-hexene forms (3S,4S)-4-bromo-3-hexanol and (3R,4R)-4-bromo-3-hexanol when it reacts with Br2 and H2O?
Problem 80b
Propose a mechanism for each of the following reactions:
b.
Problem 81a
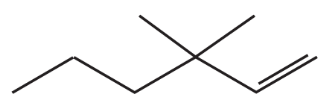
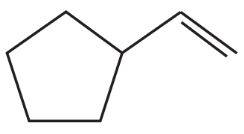
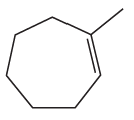
What is the major product of each of the following reactions?
a.
Problem 81b
What is the major product of each of the following reactions?
b.
Problem 82c
Draw the products of the following reactions. If the products can exist as stereoisomers, show which stereoisomers are formed
c. 1-ethylcyclohexene + H2O + H2SO4
Problem 82f
Draw the products of the following reactions. If the products can exist as stereoisomers, show which stereoisomers are formed
f. 1,2-dideuteriocyclohexene + H2, Pd/C
Problem 82i
Draw the products of the following reactions. If the products can exist as stereoisomers, show which stereoisomers are formed.
i. (Z)-3,4-dimethyl-3-heptene + H2, Pd/C
Problem 83
a. What product is obtained from the reaction of HCl with 1-butene? With 2-butene?
b. Which of the two reactions has the greater free energy of activation?
c. Which compound reacts more rapidly with HCl: (Z)-2-butene or (E)-2-butene?
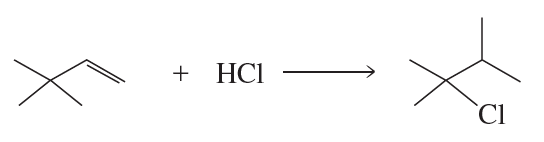
Problem 84c,d
What is the major product of the reaction of each of the following with HBr?
c.
d.
Problem 85a
For each compound, show the products obtained from ozonolysis, followed by treatment with dimethyl sulfide.
a.