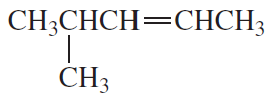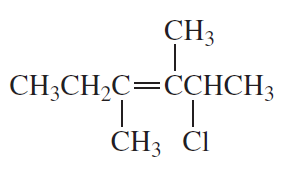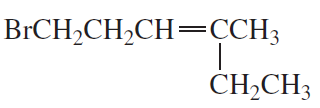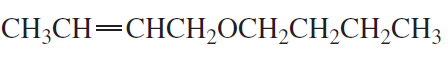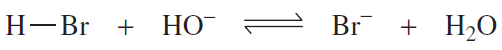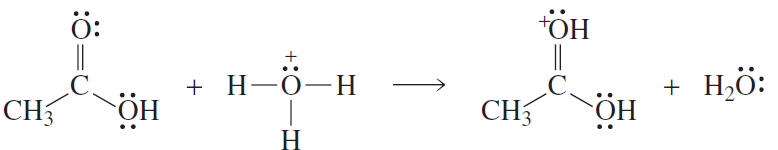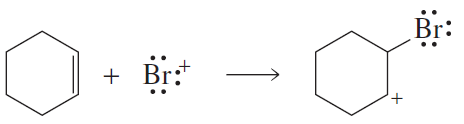Back
Back Bruice 8th Edition
Bruice 8th Edition Ch.5 - Alkenes:Structure, Nomenclature, and an Introduction to Reactivity Thermodynamics and Kinetics
Ch.5 - Alkenes:Structure, Nomenclature, and an Introduction to Reactivity Thermodynamics and KineticsProblem 1
What is the molecular formula for a 5-carbon hydrocarbon with one bond and one ring?
Problem 2(b)
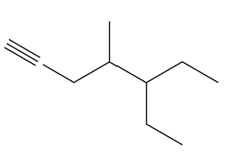
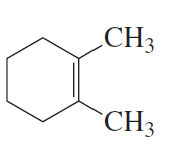
Name the following:
b.
Problem 2a
What is the molecular formula for each of the following?
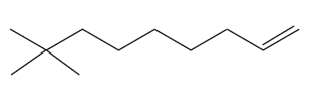
a. a 4-carbon hydrocarbon with two bonds and no rings
Problem 2b
What is the molecular formula for each of the following?
b. a 10-carbon hydrocarbon with one bond and 2 rings
Problem 4c,d
Determine the degree of unsaturation for hydrocarbons with the following molecular formulas:
c. C12H20
d. C40H56
Problem 5a
Determine the degree of unsaturation and then draw possible structures for noncyclic compounds with the following molecular formulas:
a. C3H6
Problem 5b
Determine the degree of unsaturation and then draw possible structures for noncyclic compounds with the following molecular formulas:
b. C3H4
Problem 6a
Several studies have shown that β-carotene, a precursor of vitamin A, may play a role in preventing cancer. β-Carotene has a molecular formula of C40H56, and it contains two rings and no triple bonds. How many double bonds does it have?
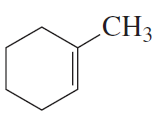
Problem 7a,b
What is each compound's systematic name?
a.
b.
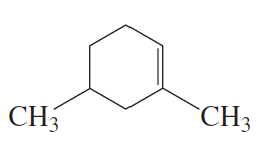
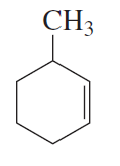
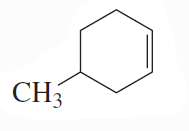
Problem 7c,d
What is each compound's systematic name?
c.
d.
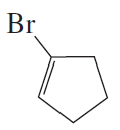
Problem 7e,f
What is each compound's systematic name?
e.
f.
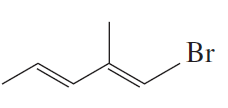
Problem 7g,h
What is each compound's systematic name?
g.
h.
Problem 8a
a. How many vinylic hydrogens does cyclopentene have?
b. How many allylic hydrogens does it have?
Problem 9a,b
Draw the structure for each of the following:
a. 3,3-dimethylcyclopentene
b. 6-bromo-2,3-dimethyl-2-hexene
Problem 9c,d
Draw the structure for each of the following:
c. ethyl vinyl ether
d. allyl alcohol
Problem 10
How many carbons are in the planar double-bond system in the following compound?
Problem 11
How many carbons are in the planar double-bond system in each of the following compounds?
a.
b.
c.
Problem 12a
Draw the isomers for the following compounds and then name each one:
a. 2-methyl-2,4-hexadiene
Problem 12b
Draw the isomers for the following compounds and then name each one:
b. 2,4-heptadiene
Problem 12c
Draw the isomers for the following compounds and then name each one:
c. 1,3-pentadiene
Problem 14
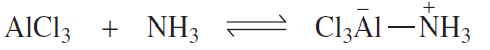
Identify the nucleophile and the electrophile in the following acid–base reactions:
a.
b.
Problem 15a,b
Use curved arrows to show the movement of electrons in the following reaction steps
a.
b.
Problem 16a,b
For each of the reactions in Problem 15, indicate which reactant is the nucleophile and which is the electrophile.
a.
b.
Problem 18b
Calculate ∆G° for the conversion of “axial” methylcyclohexane to “equatorial” methylcyclohexane at 25 °C.
Problem 19
The ∆G° for conversion of “axial” fluorocyclohexane to “equatorial” fluorocyclohexane at 25 °C is -0.25kcal/mol. Calculate the percentage of fluorocyclohexane molecules that have the fluoro substituent in an equatorial position at equilibrium.
Problem 20a
Calculate the percentage of isopropylcyclohexane molecules that have the isopropyl substituent in an equatorial position at equilibrium. (Its ∆G° value at 25 °C is -2.1 kcal/mol.)
Problem 20b
Why is the percentage of molecules with the substituent in an equatorial position greater for isopropylcyclohexane than for fluorocyclohexane?
Problem 21
a. For which reaction in each set will ∆S° be more significant?
b. For which reaction will ∆S° be positive?
1. A ⇌ B or A + B ⇌ C
2. A ⇌ B + C or A + B ⇌ C + D
Problem 22
a. For a reaction with ∆H° = -12 kcal/mol and ∆S° = 0.01 kcal mol-1 K-1, calculate the ∆G° and the equilibrium constant at:
1. 30 °C and 2. 150 °C.
b. How does ∆G° change as T increases?
c. How does Keq change as T increases?
Problem 24a
What alkene would you start with if you wanted to synthesize
a. pentane?