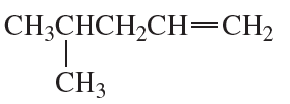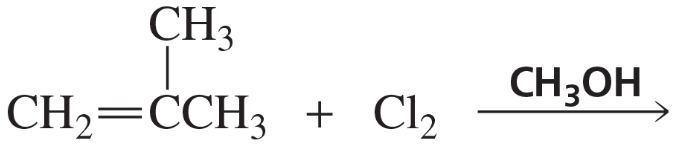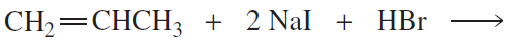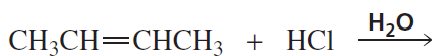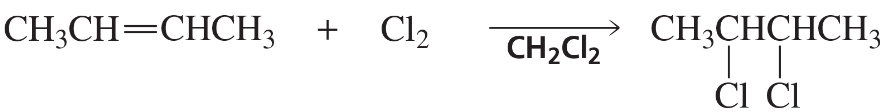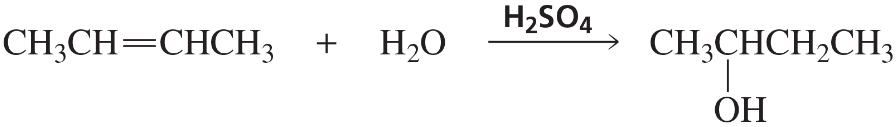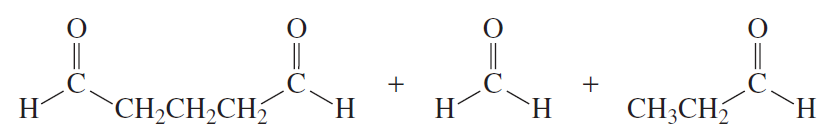Back
Back Bruice 8th Edition
Bruice 8th Edition Ch.6 - The Reactions of Alkenes The Stereochemistry of Addition Reactions
Ch.6 - The Reactions of Alkenes The Stereochemistry of Addition ReactionsProblem 17b
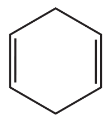
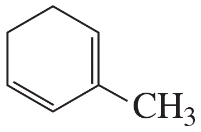
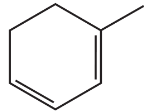
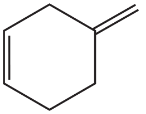
What is the major product obtained from the reaction of HBr with each of the following?
b.
Problem 18
Which is more highly regioselective: reaction of an alkene with BH3 or with 9-BBN?
Problem 19a
What is the major product obtained from hydroboration–oxidation of the following alkenes?
a. 2-methyl-2-butene
Problem 20
What will be the product of the preceding reaction if HBr is used in place of Br2?
Problem 21a
How does the first step in the reaction of propene with Br2 differ from the first step in the reaction of propene with HBr?
Problem 21b
To understand why Br− adds to a carbon of the bromonium ion rather than to the positively charged bromine, draw the product that would be obtained if Br− did add to bromine.
Problem 22
Why are Na+ and K+ unable to form covalent bonds?
Problem 23a
Each of the following reactions has two nucleophiles that could add to the intermediate formed by the reaction of the alkene with an electrophile. What is the major product of each reaction?
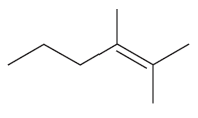
a.
Problem 23b
Each of the following reactions has two nucleophiles that could add to the intermediate formed by the reaction of the alkene with an electrophile. What is the major product of each reaction?
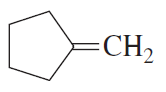
b.
Problem 23c
Each of the following reactions has two nucleophiles that could add to the intermediate formed by the reaction of the alkene with an electrophile. What is the major product of each reaction?
c.
Problem 24
What is the product of the addition of I—Cl to 1-butene? (Hint: Chlorine is more electronegative than iodine [Table 1.3].)
Problem 25a,b
What will be the major product obtained from the reaction of Br2 with 1-butene if the reaction is carried out in
a. dichloromethane?
b. water?
Problem 25c,d
What will be the major product obtained from the reaction of Br2 with 1-butene if the reaction is carried out in
c. ethyl alcohol?
d. methyl alcohol?
Problem 27a,b
Draw structures for the following:
a. 2-propyloxirane
b. cyclohexene oxide
Problem 27c
Draw structures for the following:
c. 2,2,3,3-tetramethyloxirane
Problem 27d
Draw structures for the following:
d. 2,3-epoxy-2-methylpentane
Problem 28a,b
What alkene would you treat with a peroxyacid in order to obtain each of the epoxides in Problem 27?
a. 2-propyloxirane
b. cyclohexene oxide
Problem 28c.d
What alkene would you treat with a peroxyacid in order to obtain each of the epoxides in Problem 27?
c. 2,2,3,3-tetramethyloxirane
d. 2,3-epoxy-2-methylpentane
Problem 29a,b
Identify each of the following reactions as an oxidation reaction, a reduction reaction, or neither.
a.
b.
Problem 30a
What products are formed when the following compounds react with ozone and then with dimethyl sulfide?
a.
Problem 30b
What products are formed when the following compounds react with ozone and then with dimethyl sulfide?
b.
Problem 30c,d
What products are formed when the following compounds react with ozone and then with dimethyl sulfide?
c.
d.
Problem 30e,f
What products are formed when the following compounds react with ozone and then with dimethyl sulfide?
e.
f.
Problem 31a
What alkene would give only a ketone with three carbons as a product of oxidative cleavage?
Problem 31b
What alkenes would give only an aldehyde with four carbons as a product of oxidative cleavage
Problem 32
What aspect of the structure of the alkene does ozonolysis not tell you?
Problem 33b
The following products were obtained from the oxidative cleavage of a diene. What is the structure of the diene?
Problem 34a
The following product was obtained from the ozonolysis of an alkene followed by treatment with dimethyl sulfide. What is the structure of the alkene?
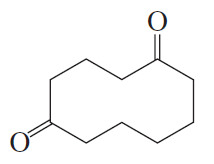
a.
Problem 34b
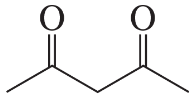
The following product was obtained from the ozonolysis of an alkene followed by treatment with dimethyl sulfide. What is the structure of the alkene?
b.
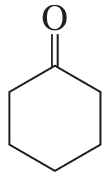
Problem 34c
The following product was obtained from the ozonolysis of an alkene followed by treatment with dimethyl sulfide. What is the structure of the alkene?
c.