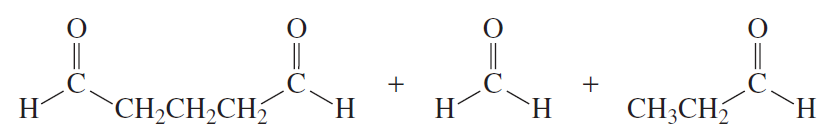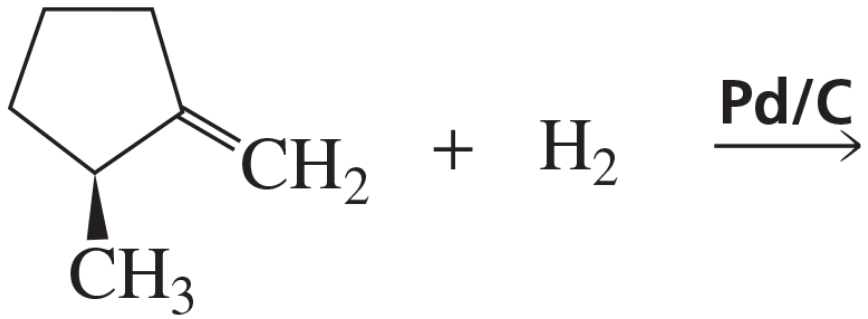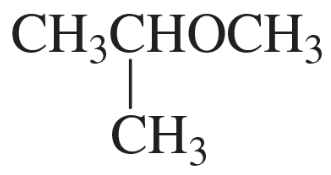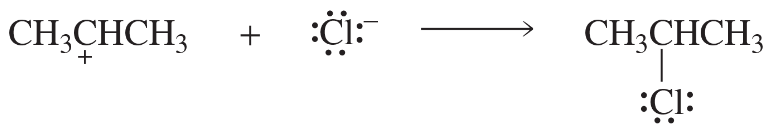Back
Back Bruice 8th Edition
Bruice 8th Edition Ch.6 - The Reactions of Alkenes The Stereochemistry of Addition Reactions
Ch.6 - The Reactions of Alkenes The Stereochemistry of Addition ReactionsProblem 32
What aspect of the structure of the alkene does ozonolysis not tell you?
Problem 33b
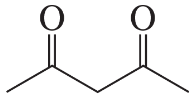
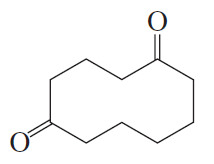
The following products were obtained from the oxidative cleavage of a diene. What is the structure of the diene?
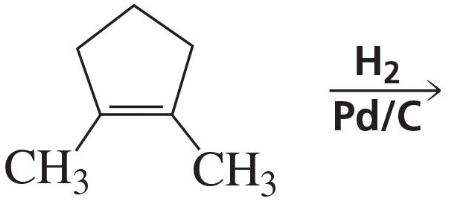
Problem 34a
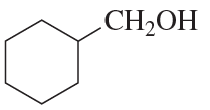
The following product was obtained from the ozonolysis of an alkene followed by treatment with dimethyl sulfide. What is the structure of the alkene?
a.
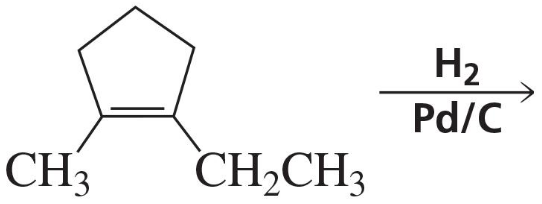
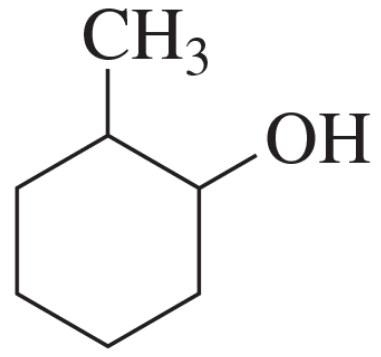
Problem 34b
The following product was obtained from the ozonolysis of an alkene followed by treatment with dimethyl sulfide. What is the structure of the alkene?
b.
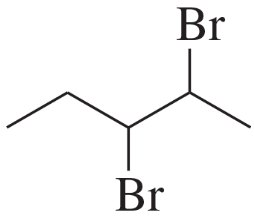
Problem 34c
The following product was obtained from the ozonolysis of an alkene followed by treatment with dimethyl sulfide. What is the structure of the alkene?
c.
Problem 35
What characteristics must the reactant of a stereospecific reaction have?
Problem 36a,b,c
a. Is the reaction of 2-butene with HBr regioselective?
b. Is it stereoselective?
c. Is it stereospecific?
Problem 36d,e,f
d. Is the reaction of 1-butene with HBr regioselective?
e. Is it stereoselective?
f. Is it stereospecific?
Problem 37b
What stereoisomers are obtained from each of the following reactions?
b.
Problem 40
a. What stereoisomers are formed in the following reaction? Are they enantiomers or diastereomers?
b. Which stereoisomer is formed in greater yield?
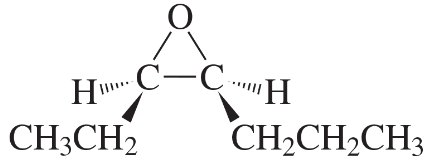
Problem 41(1)
a. What alkene is required to synthesize each of the following compounds?
b. What other epoxide is formed in each synthesis?
c. Assign an R or S configuration to each asymmetric center.
1.
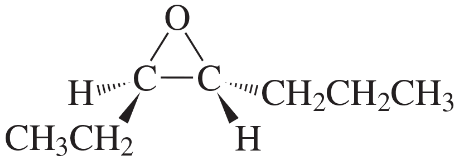
Problem 41(2)
a. What alkene is required to synthesize each of the following compounds?
b. What other epoxide is formed in each synthesis?
c. Assign an R or S configuration to each asymmetric center.
2.
Problem 42a,b
What stereoisomers are obtained from hydroboration–oxidation of the following compounds? Assign an R or S configuration to each asymmetric center.
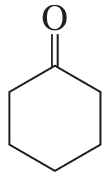
a. cyclohexene
b. 1-ethylcyclohexene
Problem 42c
What stereoisomers are obtained from hydroboration–oxidation of the following compounds? Assign an R or S configuration to each asymmetric center.
c. cis-2-butene
Problem 42d
What stereoisomers are obtained from hydroboration–oxidation of the following compounds? Assign an R or S configuration to each asymmetric center.
d. (Z)-3,4-dimethyl-3-hexene
Problem 43
The reaction of 2-ethyl-1-pentene with Br2, with H2 + Pd/C, or with R2BH/THF followed by aqueous HO- + H2O2 leads to a racemic mixture. Explain why a racemic mixture is obtained in each case.
Problem 44
Using a sample of trans-2-pentene, how could you prove that the addition of Br2 forms a cyclic bromonium ion intermediate rather than a carbocation intermediate?
Problem 45c,d
What stereoisomers are obtained from the following reactions?
c. (E)-3-methyl-2-pentene + HBr
d. cis-3-hexene + HBr
Problem 46
When Br2 adds to a cis alkene that has different substituents attached to each of the two sp2 carbons, such as cis-2-heptene, identical amounts of the two threo enantiomers are obtained even though Br- is more likely to add to the less sterically hindered carbon of the bromonium ion. Explain why identical amounts of the two enantiomers are obtained.
Problem 49c,d
What stereoisomers would you expect to obtain from each of the following reactions?
c.
d.
Problem 50
a. What is the major product obtained from the reaction of propene and Br2 plus excess Cl-?
b. Indicate the relative amounts of the stereoisomers that are obtained.
Problem 51b
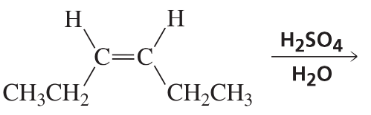
What is the product of the reaction of maleate and H2O when H2SO4 is used as a catalyst instead of fumarase?
Problem 53a
Show how each of the following compounds can be synthesized from an alkene:
a.
Problem 53b
Show how each of the following compounds can be synthesized from an alkene:
b.
Problem 53c
Show how each of the following compounds can be synthesized from an alkene:
c.
Problem 54
Explain why 3-methylcyclohexene should not be used as the starting material in Problem 52b.
Problem 56a
Which electrophilic addition reactions
a. form a carbocation intermediate? intermediate?
Problem 56c
Which electrophilic addition reactions
c. form a three-membered ring
Problem 56d
Which electrophilic addition reactions
d. form a five-membered ring intermediate?
Problem 57a
Identify the electrophile and the nucleophile in each of the following reaction steps and then draw curved arrows to illustrate the bond-making and bondbreaking processes.
a.