Are one or more of the disaccharides maltose, lactose, cellobiose, and sucrose part of the trisaccharide in Problem 20.23? If so, identify which disaccharide and its location. (Hint: Look for an α-1,4 link, β-1,4 link, or 1,2 link, and then determine if the correct monosaccharides are present.)
Ch.20 Carbohydrates

Chapter 20, Problem 31d
Classify the four carbohydrates (a)–(d) by indicating the nature of the carbonyl group and the number of carbon atoms present. For example, glucose is an aldohexose.
d. 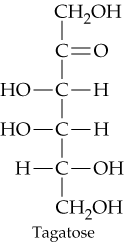
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the classification of carbohydrates. Carbohydrates are classified based on two main criteria: (1) the type of carbonyl group present (aldehyde or ketone), and (2) the number of carbon atoms in the molecule. For example, an 'aldohexose' is a carbohydrate with an aldehyde group and six carbon atoms.
Step 2: Examine the structure of each carbohydrate provided in the problem. Look for the functional group that contains the carbonyl (C=O). If the carbonyl group is at the end of the chain, it is an aldehyde, and the carbohydrate is classified as an 'aldo-'. If the carbonyl group is within the chain, it is a ketone, and the carbohydrate is classified as a 'keto-'.
Step 3: Count the number of carbon atoms in the structure of each carbohydrate. This will determine the suffix of the classification. For example, 3 carbons = triose, 4 carbons = tetrose, 5 carbons = pentose, and 6 carbons = hexose.
Step 4: Combine the information from Steps 2 and 3 to classify each carbohydrate. For example, if a carbohydrate has an aldehyde group and six carbon atoms, it is an aldohexose. If it has a ketone group and five carbon atoms, it is a ketopentose.
Step 5: Repeat the process for all four carbohydrates (a)–(d) provided in the problem. Carefully analyze each structure to determine the nature of the carbonyl group and the number of carbon atoms, then assign the appropriate classification.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Carbonyl Group
The carbonyl group is a functional group characterized by a carbon atom double-bonded to an oxygen atom (C=O). In carbohydrates, the nature of the carbonyl group determines whether the sugar is classified as an aldose (with an aldehyde group at the end of the carbon chain) or a ketose (with a ketone group within the carbon chain). This classification is essential for identifying the type of carbohydrate.
Recommended video:
Guided course
Functional Groups with Carbonyls Example 3
Number of Carbon Atoms
The number of carbon atoms in a carbohydrate is crucial for its classification. Carbohydrates are often categorized based on the number of carbon atoms they contain, such as triose (3), tetrose (4), pentose (5), hexose (6), and so on. This classification helps in understanding the structure and function of the carbohydrate, as different carbon counts can lead to different properties and biological roles.
Recommended video:
Guided course
Amino Acid Catabolism: Carbon Atoms Concept 2
Carbohydrate Classification
Carbohydrates are classified into simple sugars (monosaccharides) and complex carbohydrates (oligosaccharides and polysaccharides). Monosaccharides can be further classified based on the type of carbonyl group and the number of carbon atoms. Understanding this classification is essential for analyzing the structure and reactivity of carbohydrates, as well as their roles in biological systems.
Recommended video:
Guided course

Classification of Carbohydrates Example 1
Related Practice
Textbook Question
Textbook Question
In solution, glucose exists predominantly in the cyclic hemiacetal form, which does not contain an aldehyde group. How is it possible for mild oxidizing agents to oxidize glucose?
Textbook Question
Classify the four carbohydrates (a)–(d) by indicating the nature of the carbonyl group and the number of carbon atoms present. For example, glucose is an aldohexose.
c.
Textbook Question
How many chiral carbon atoms are present in each of the molecules shown in Problem 20.31?
a.
b.
c.
d.
Textbook Question
Draw the open-chain structure of a 4-carbon deoxy sugar.
Textbook Question
Name four important monosaccharides and tell where each occurs in nature.
