Textbook Question
Propanamide and methyl acetate have about the same molar mass, both are quite soluble in water, and yet the boiling point of propanamide is 213 °C, whereas that of methyl acetate is 57 °C. Explain.

 Verified step by step guidance
Verified step by step guidance
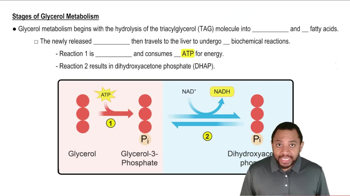
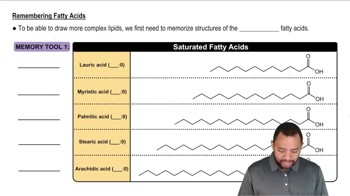
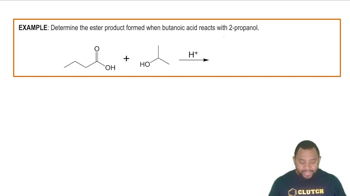
Propanamide and methyl acetate have about the same molar mass, both are quite soluble in water, and yet the boiling point of propanamide is 213 °C, whereas that of methyl acetate is 57 °C. Explain.
Each of the following materials has an ester that is responsible for its smell and/or flavor. Search the internet and determine what that ester is, draw its structure, and what carboxylic acid and alcohol are used to form it.
a. Juicy Fruit gum flavoring
What is the difference between a phosphate diester and an ester of a diphosphate? Give an example of each.
Mention at least two simple chemical tests by which you can distinguish between benzaldehyde and benzoic acid.
Draw all possible carboxylic acids with the formula C5H10O2.