Propanamide and methyl acetate have about the same molar mass, both are quite soluble in water, and yet the boiling point of propanamide is 213 °C, whereas that of methyl acetate is 57 °C. Explain.
Ch.17 Carboxylic Acids and Their Derivatives

Chapter 17, Problem 75
What is the difference between a phosphate diester and an ester of a diphosphate? Give an example of each.
 Verified step by step guidance
Verified step by step guidance1
Understand the terminology: A phosphate diester contains two ester bonds to phosphate, meaning the phosphate group is bonded to two organic groups via oxygen atoms. An ester of a diphosphate, on the other hand, involves a diphosphate group (two phosphate groups linked by an oxygen atom) where one of the phosphate groups forms an ester bond with an organic group.
For a phosphate diester, consider the general structure: \( R-O-PO(OH)-O-R' \), where \( R \) and \( R' \) are organic groups. An example is DNA, where the phosphate group forms diester bonds with the sugar molecules in the backbone.
For an ester of a diphosphate, consider the general structure: \( R-O-PO_3-O-PO_3^{2-} \), where \( R \) is an organic group. An example is adenosine diphosphate (ADP), where the adenosine molecule is esterified to a diphosphate group.
Compare the two structures: In a phosphate diester, the phosphate group is central and forms two ester bonds. In an ester of a diphosphate, the diphosphate group is central, and only one of the phosphate groups forms an ester bond.
Summarize the key difference: A phosphate diester involves a single phosphate group with two ester bonds, while an ester of a diphosphate involves a diphosphate group with one ester bond to an organic molecule.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Phosphate Diester
A phosphate diester is a chemical compound where two alcohol groups are esterified with a phosphate group. This structure is commonly found in nucleic acids, such as DNA and RNA, where the phosphate backbone connects the sugar molecules through diester linkages. The presence of two ester bonds distinguishes it from simple esters.
Recommended video:
Guided course

Naming Ionic Compounds
Ester of a Diphosphate
An ester of a diphosphate involves a phosphate group bonded to two other phosphate groups through ester linkages. This structure is typically seen in molecules like adenosine triphosphate (ATP), where the energy currency of the cell is stored in the high-energy bonds between the phosphate groups. The diphosphate structure indicates the presence of two phosphate units.
Recommended video:
Guided course
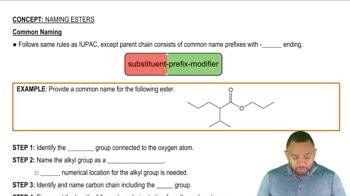
Common Naming: Esters Concept 2
Chemical Structure and Function
Understanding the chemical structure and function of phosphate diesters and diphosphate esters is crucial for grasping their roles in biochemistry. The arrangement of phosphate groups and their esterification with alcohols influence their reactivity and biological functions, such as energy transfer in ATP or the stability of nucleic acids. This knowledge is essential for studying metabolic pathways and molecular biology.
Recommended video:
Guided course

Logarithmic Functions
Related Practice
Textbook Question
Textbook Question
In the following compound
a. Identify the phosphate ester linkage.
Textbook Question
In the following compound
b. Identify the phosphate anhydride linkage.
Textbook Question
Cyclic ribose nucleotide phosphates, such as cyclic AMP (cAMP), are important signaling agents in living cells; all have the general structure shown here. What kind of linkage holds the phosphate to the ribose (see arrows; ribose is highlighted in blue)?
Textbook Question
Mention at least two simple chemical tests by which you can distinguish between benzaldehyde and benzoic acid.
Textbook Question
Write the formula of the triester formed from glycerol and stearic acid.
