Textbook Question
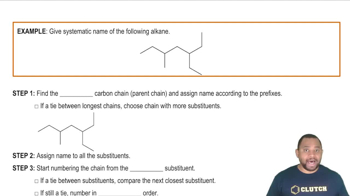
Draw structures corresponding to the following IUPAC names:
a. trans-2-Pentene
b. trans-3,4-Dimethyl-3-hexene
c. 2-Methyl-1,3-butadiene
d. trans-3-Heptene

 Verified step by step guidance
Verified step by step guidance
Draw structures corresponding to the following IUPAC names:
a. trans-2-Pentene
b. trans-3,4-Dimethyl-3-hexene
c. 2-Methyl-1,3-butadiene
d. trans-3-Heptene
Seven alkynes have the formula C6H10. Draw them using line structures.
Draw and name all phenols with the formula C7H8O .
If 2-methyl-2-pentene were converted into 1-hexene, what kind of reaction would that be?
If bromocyclohexane were converted into cyclohexene, what kind of reaction would that be?