Textbook Question
Identify the functional groups in the following molecules:
(b)
1
views

 Verified step by step guidance
Verified step by step guidance

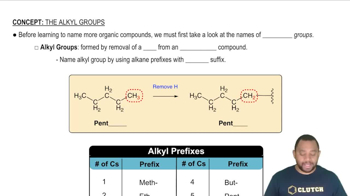
Identify the functional groups in the following molecules:
(b)
What characteristics of carbon make possible the existence of so many different organic compounds?
Why are most organic compounds nonconducting and insoluble in water?
For each of the following, give an example of a member compound containing 5 carbons total:
(a) Alcohol
Propose structures for molecules that fit the following descriptions:
(b) An ester with the formula C6H12O2
Propose structures for molecules that fit the following descriptions:
(c) A compound with the formula C3H7NOS that is both an amide and a thiol