Textbook Question
Which of the following processes results in an increase in entropy of the system?
a. A drop of ink spreading out when it is placed in water
b. Steam condensing into drops on windows
c. Constructing a building from loose bricks

 Verified step by step guidance
Verified step by step guidance
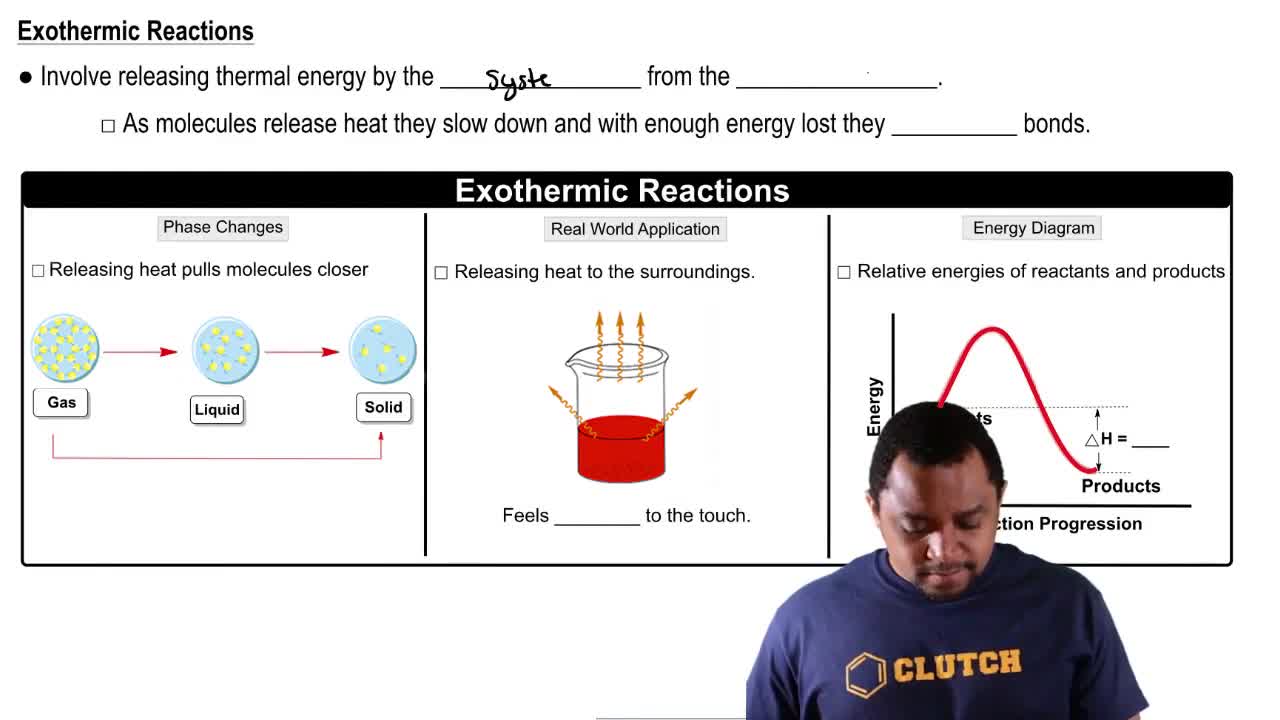
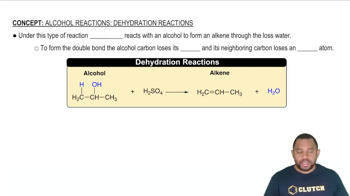
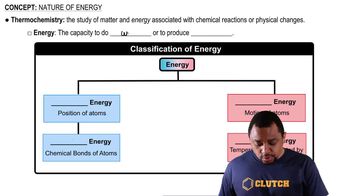
Which of the following processes results in an increase in entropy of the system?
a. A drop of ink spreading out when it is placed in water
b. Steam condensing into drops on windows
c. Constructing a building from loose bricks
For each of the following processes, specify whether entropy increases or decreases. Explain each of your answers.
a. Assembling a jigsaw puzzle
What two factors affect the spontaneity of a reaction?
Under what conditions might a reaction be endothermic but exergonic? Explain.
For the reaction
b. Does entropy increase or decrease in this process?
For the reaction 2 Hg(l) + O2(g) → 2 HgO(s), ∆H = –43 kcal/mol (–180 kJ/mol).
a. Does entropy increase or decrease in this process? Explain.