Textbook Question
Calcium citrate, Ca(C6H5O7)2(MW = 498.5 amu), is a common dietary supplement to provide calcium needed for strong teeth and bones.
b. What mass of calcium citrate would be needed to provide the recommended daily intake of calcium?

 Verified step by step guidance
Verified step by step guidance
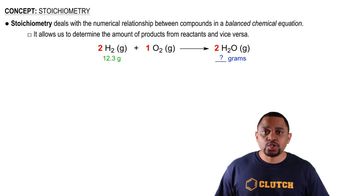
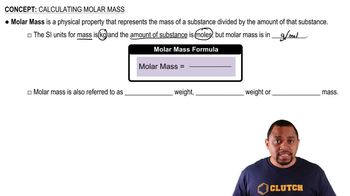
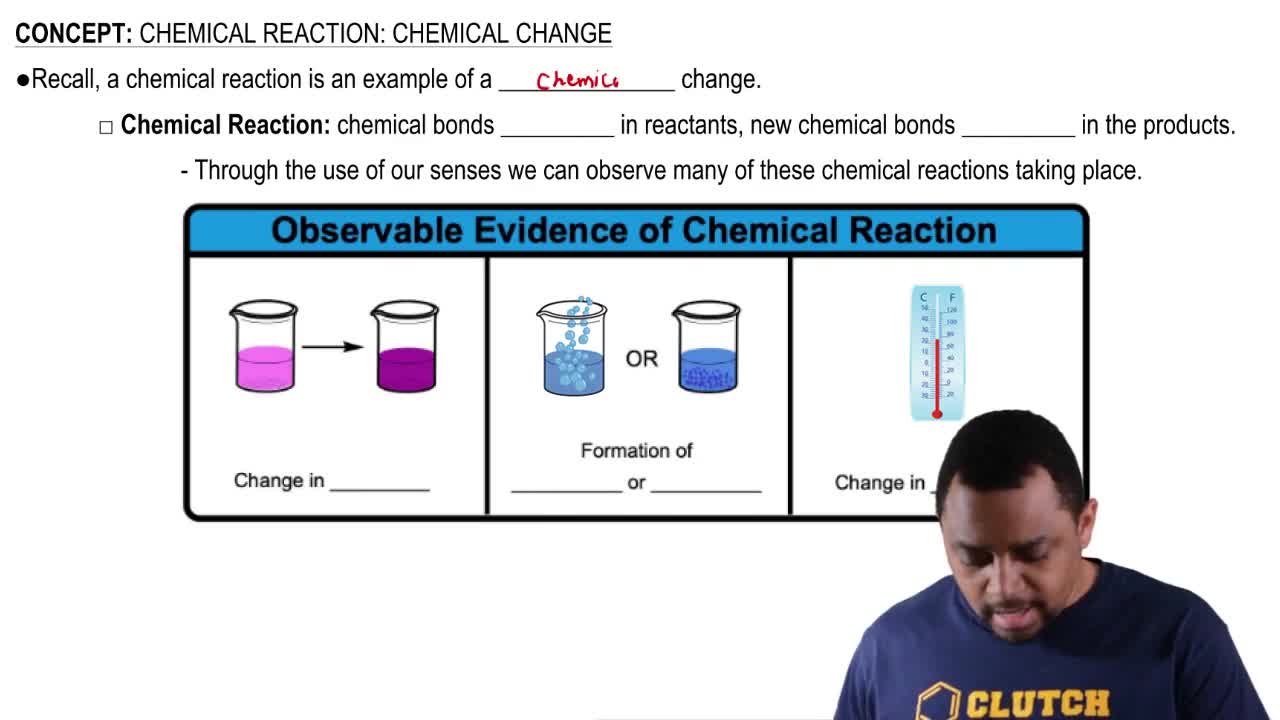
Calcium citrate, Ca(C6H5O7)2(MW = 498.5 amu), is a common dietary supplement to provide calcium needed for strong teeth and bones.
b. What mass of calcium citrate would be needed to provide the recommended daily intake of calcium?
Obtain a bottle of aspirin and identify the amount of active ingredient (acetylsalicylic acid, C9H8O4) per tablet.
a. How many moles of aspirin are in one tablet?
Lovastatin, a drug used to lower serum cholesterol.
a. Look up the molecular formula for Lovastatin and calculate the molar mass.