Textbook Question
Which of the following contains a coordinate covalent bond? (Hint: How many covalent bonds would you expect the central atom (underlined/bold) to form?)
a. PbCl2
b. Cu(NH3)42+
c. NH4+

 Verified step by step guidance
Verified step by step guidance
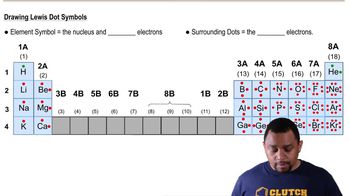

Which of the following contains a coordinate covalent bond? (Hint: How many covalent bonds would you expect the central atom (underlined/bold) to form?)
a. PbCl2
b. Cu(NH3)42+
c. NH4+
What are likely formulas for the following molecules?
a. CH2Cl?
b. BH?
c. NI?
d. SiCl?
What is a coordinate covalent bond, and how does it differ from a covalent bond?
Identify the bonds formed between the following pairs of atoms as either covalent or ionic.
d. Zinc and fluorine
A compound of gallium with chlorine has a melting point of 77°C and a boiling point of 201°C. Is the compound ionic or covalent? What is a likely formula?
Distinguish between the following:
b. A structural formula and a condensed structure