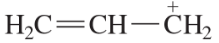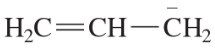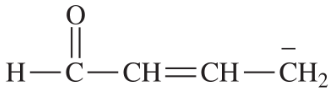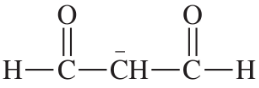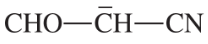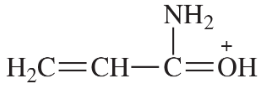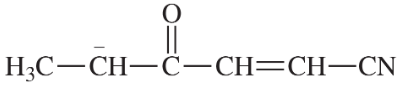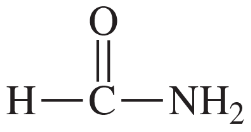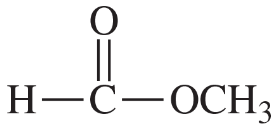Back
BackProblem 1.15
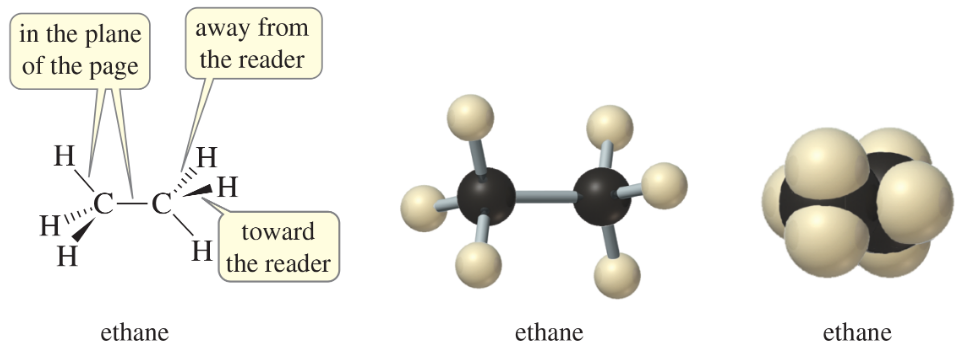
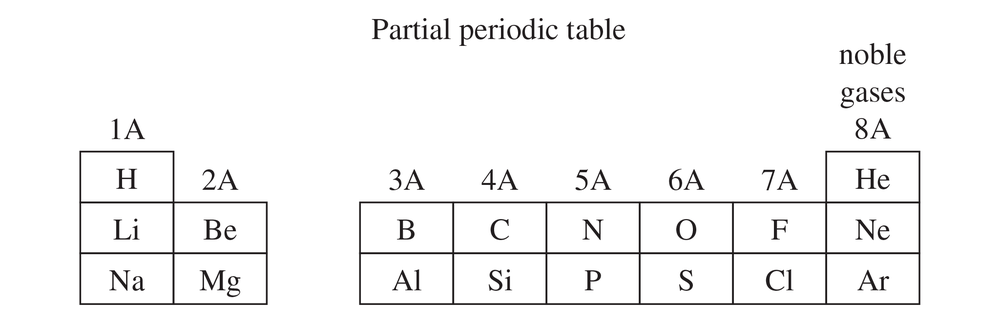
Use your molecular models to make ethane, and compare the model with the preceding structures.
- For each of the following compounds, 1. Give the hybridization and approximate bond angles around each atom except hydrogen. 2. Draw a three-dimensional diagram, including any lone pairs of electrons. d. (CH3)3N e. [CH3NH3]+ f. CH3COOH
Problem 1
Problem 1a
Nitrogen has relatively stable isotopes (half-life greater than 1 second) of mass numbers 13, 14, 15, 16, and 17. All except 14N and 15N are radioactive.) Calculate how many protons and neutrons are in each of these isotopes of nitrogen.
Problem 1b
Write the electronic configurations of the third-row elements shown in the partial periodic table in Figure 1-6.
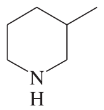
Problem 2e,f,g
Draw Lewis structures for the following compounds.
e. dimethylamine, CH3NHCH3
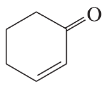
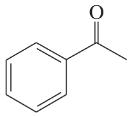
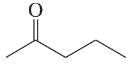
f. diethyl ether, CH3CH2OCH2CH3
g. 1-chloropropane,CH3CH2CH2Cl
Problem 3,4i-k
Write Lewis structures for the following molecular formulas. Circle any lone pairs (pairs of nonbonding electrons) in the structures.
i. C3H6 (one double bond)
j. C3H4 (two double bonds)
k. C3H4 (one triple bond)
Problem 5f-j
Use electronegativities to predict the direction of the dipole moments of the following bonds.
(f) N—Cl
(g) N—O
(h) N—S
(i) N—B
(j) B—Cl
Problem 6e-h
Draw Lewis structures for the following compounds and ions, showing appropriate formal charges.
(e) +CH3
(f) –CH3
(g) NaBH4
(h) NaBH3CN
Problem 6i-l
Draw Lewis structures for the following compounds and ions, showing appropriate formal charges.
(i) (CH3)2O—BF3
(j) [HONH3]+
(k) KOC(CH3)3
(l) [H2C=OH]+
Problem 7a,b,c
Draw the important resonance forms for the following molecules and ions.
(a) CO32–
(b)
(c)
Problem 7d,e,f
Draw the important resonance forms for the following molecules and ions.
(d) NO3–
(e) NO2–
(f)
Problem 8c
For each of the following compounds, draw the important resonance forms. Indicate which structures are major and minor contributors or whether they have the same energy.
(c) [H2COCH3]+
Problem 8d
For each of the following compounds, draw the important resonance forms. Indicate which structures are major and minor contributors or whether they have the same energy.
(d) [H2CNO2]–
Problem 8e,f
For each of the following compounds, draw the important resonance forms. Indicate which structures are major and minor contributors or whether they have the same energy.
(e) [CH3C(OH)2]+
(f) [CH2CHNH]–
Problem 8g,h
For each of the following compounds, draw the important resonance forms. Indicate which structures are major and minor contributors or whether they have the same energy.
(g)
(h)
Problem 9c,d
Draw the important resonance forms of the following cations and anions:
(c)
(d)
Problem 10c
Use resonance structures to identify the areas of high and low electron density in the following compounds:
(c)
Problem 10d
Use resonance structures to identify the areas of high and low electron density in the following compounds:
(d)
Problem 11d,e
Draw complete Lewis structures for the following condensed structural formulas.
(d) CH2CHCHO
(e) (CH3)3CCOCHCH2
Problem 12a,b
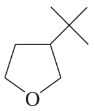
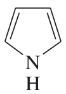
Give Lewis structures corresponding to the following line–angle structures. Give the molecular formula for each structure.
(a)
(b)
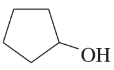
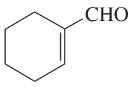
Problem 12c,d
Give Lewis structures corresponding to the following line–angle structures. Give the molecular formula for each structure.
(c)
(d)
Problem 12e,f
Give Lewis structures corresponding to the following line–angle structures. Give the molecular formula for each structure.
(e)
(f)
Problem 12g,h
Give Lewis structures corresponding to the following line–angle structures. Give the molecular formula for each structure.
(g)
(h)
Problem 13a,b
Draw line-angle structures for the compounds (a) through (h).
a. CH3(CH2)3CH(CH3)2
b. (CH3)2CHCH2Cl
Problem 13c,d
Draw line-angle structures for the compounds (a) through (h).
c. CH3CH2COCN
d. CH2CHCHO
Problem 13e,f
Draw line-angle structures for the compounds (a) through (h).
e. (CH3)3CCOCHCH2
f. CH3COCOOH
Problem 13g,h
Draw line-angle structures for the compounds (a) through (h).
g. (CH3CH2)2CO
h. (CH3)3COH
Problem 14a
Compute the empirical and molecular formulas for each of the following elemental analyses. In each case, propose at least one structure that fits the molecular formula.
Problem 14b
Compute the empirical and molecular formulas for each of the following elemental analyses. In each case, propose at least one structure that fits the molecular formula.
Problem 14c
Compute the empirical and molecular formulas for each of the following elemental analyses. In each case, propose at least one structure that fits the molecular formula.