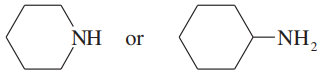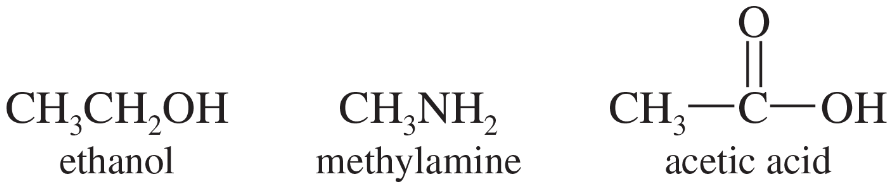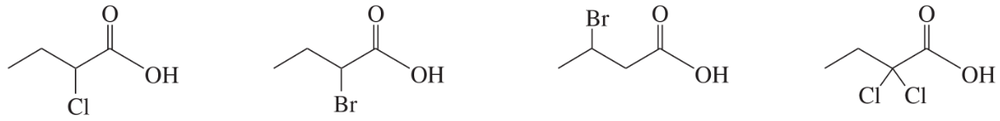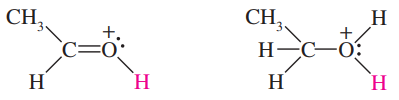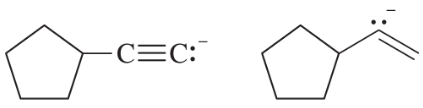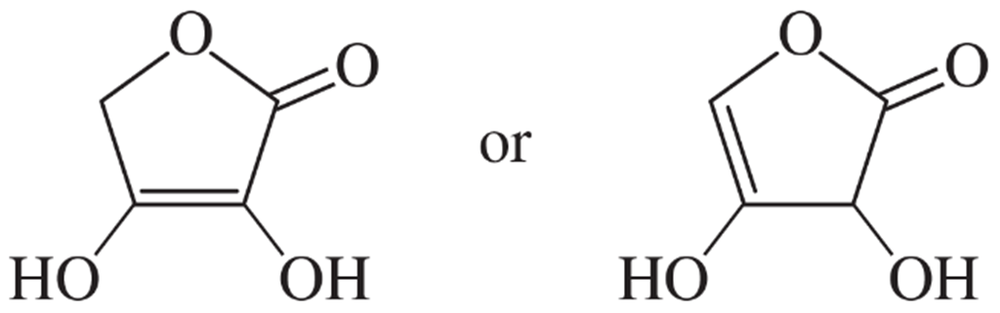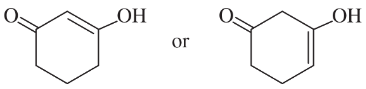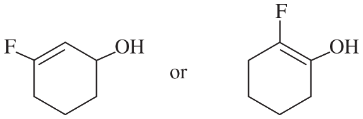Back
BackProblem 1a
The C=O double bond has a dipole moment of about 2.4 D and a bond length of about 1.23 Å.
a. Calculate the amount of charge separation in this bond.
Problem 1b
The C=O double bond has a dipole moment of about 2.4 D and a bond length of about 1.23 Å.
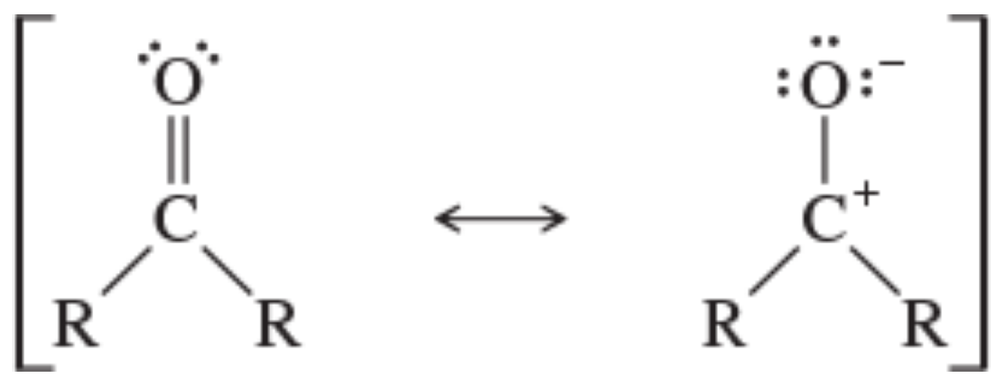
b. Use this information to evaluate the relative importance of the following two resonance contributors:
Problem 2a
The N—F bond is more polar than the N—H bond, but NF3 has a smaller dipole moment than NH3. Explain this curious result.
NF3
μ= 0.2 D
NH3
μ = 1.5 D
Problem 3d,e,f
1. Draw the Lewis structure.
2. Show how the bond dipole moments (and those of any nonbonding pairs of electrons) contribute to the molecular dipole moment.
3. Estimate whether the compound will have a large, small, or zero dipole moment.
d. CH3F
e. CF4
f. CH3OH
Problem 3g,h,i
1. Draw the Lewis structure.
2. Show how the bond dipole moments (and those of any nonbonding pairs of electrons) contribute to the molecular dipole moment.
3. Estimate whether the compound will have a large, small, or zero dipole moment.
g. HCN
h. CH3CHO
i. H2C=NH
Problem 4a
Two isomers of 1,2-dichloroethene are known. One has a dipole moment of 2.4 D; the other has zero dipole moment. Draw the two isomers, and explain why one has zero dipole moment.
CHCl=CHCl 1,2-dichloroethene
Problem 5a,b
Draw the hydrogen bonding that takes place between
a. two molecules of ethanol.
b. two molecules of propylamine.
Problem 5c,d
Draw the hydrogen bonding that takes place between
c. a molecule of dimethyl ether and two molecules of water.
d. two molecules of trimethylamine and a molecule of water.
Problem 6a,b,c
For each pair of compounds, circle the compound you expect to have the higher boiling point. Explain your reasoning.
a. (CH3)3C—C(CH3)3 or (CH3)2CH—CH2CH2—CH(CH3)2
b. CH3(CH2)6CH3 or CH3(CH2)5CH2OH
c. CH3CH2OCH2CH3 or CH3CH2CH2CH2OH
Problem 6d,e,f
For each pair of compounds, circle the compound you expect to have the higher boiling point. Explain your reasoning.
(d) HOCH2—(CH2)4—CH2OH or (CH3)3CCH(OH)CH3
(e) (CH3CH2CH2)2NH or (CH3CH2)3N
(f)
Problem 7a
Circle the member of each pair that is more soluble in water.
a. CH3CH2OCH2CH3 or CH3CH2CH2CH2CH3
b. CH3CH2OCH2CH3 or CH3CH2CH2OH
c. CH3CH2NHCH3 or CH3CH2CH2CH3
d. CH3CH2OH or CH3CH2CH2CH2OH
e.
Problem 8
Calculate the pH of the following solutions.
a. 5.00 g of HBr in 100 mL of aqueous solution
b. 1.50 g of NaOH in 50 mL of aqueous solution
Problem 9b
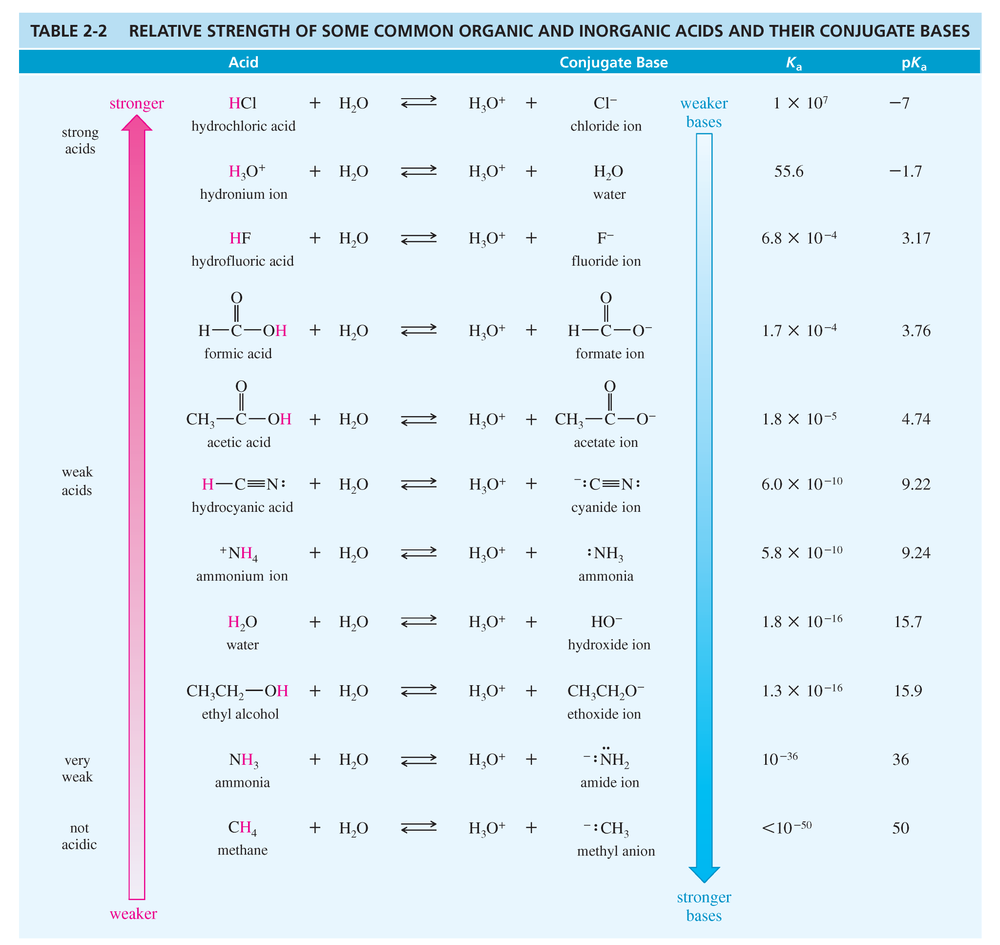
Ammonia appears in [TABLE 2-2 ] as both an acid and a conjugate base. a. Explain how ammonia can act as both an acid and a base. Which of these roles does it commonly fill in aqueous solutions?
b. Show how water can serve as both an acid and a base.
Problem 9d
Ammonia appears in [TABLE 2-2] as both an acid and a conjugate base.
d. Show how methanol (CH3OH) can serve as both an acid and a base. Write an equation for the reaction of methanol with sulfuric acid.
Problem 10a,b,c
Write equations for the following acid–base reactions. Use the information in Table 2-2 or Appendix 4 to predict whether the equilibrium will favor the reactants or the products.
a. HCOOH + –CN
b. CH3COO– + CH3OH
c. (CH3)2CHOH + NaNH2
Problem 11a
Ethanol, methylamine, and acetic acid are all amphoteric, reacting as either acids or bases depending on the conditions.
a. Rank ethanol, methylamine, and acetic acid in decreasing order of acidity. In each case, show the equation for the reaction with a generic base (B:−) to give the conjugate base.
Problem 11b
Ethanol, methylamine, and acetic acid are all amphoteric, reacting as either acids or bases depending on the conditions.
b. Rank ethanol, methylamine, and acetic acid in decreasing order of basicity. In each case, show the equation for the reaction with a generic acid (HA) to give the conjugate acid.
Problem 12a,b
For each of the following reactions, suggest which solvent(s) would be compatible with the acids and bases involved. (We will ignore any other possible reactions for now.) Your choices of solvents are pentane, diethyl ether, ethanol, water, and ammonia. Refer to Appendix 4 for any needed values of pKa, or estimate them.
a. CH3Li + H—C≡C—H → CH4 + H—C≡CLi
b. CH3Li + (CH3)3C—OH → CH4 + (CH3)3C—OLi
Problem 12c,d
For each of the following reactions, suggest which solvent(s) would be compatible with the acids and bases involved. (We will ignore any other possible reactions for now.) Your choices of solvents are pentane, diethyl ether, ethanol, water, and ammonia. Refer to Appendix 4 for any needed values of pKa, or estimate them.
c.
d.
Problem 13a,b,c
Write equations for the following acid–base reactions. Label the conjugate acids and bases, and show any inductive stabilization. Predict whether the equilibrium favors the reactants or products. Try to do this without using a table of pKa values, but if you need a hint, you can consult Appendix 4.
a. CH3CH2OH + CH3NH−
b. F3CCOONa + Br3C—COOH
c. CH3OH + H2SO4
Problem 13g,h,i
Write equations for the following acid–base reactions. Label the conjugate acids and bases, and show any inductive stabilization. Predict whether the equilibrium favors the reactants or products. Try to do this without using a table of pKa values, but if you need a hint, you can consult Appendix 4.
g. NaOCH2CH3 + Cl2CHCH2OH
h. H2Se + NaNH2
i. CH3CHFCOOH + FCH2CH2COO–
Problem 13j
Write equations for the following acid–base reactions. Label the conjugate acids and bases, and show any inductive stabilization. Predict whether the equilibrium favors the reactants or products. Try to do this without using a table of pKa values, but if you need a hint, you can consult Appendix 4.
j. CF3CH2O– + FCH2CH2OH
Problem 14a
Rank the following acids in decreasing order of their acid strength. In each case, explain why the previous compound should be a stronger acid than the one that follows it.
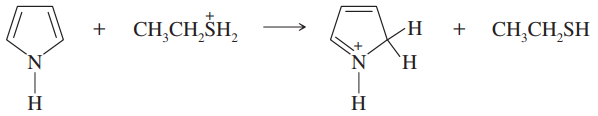
Problem 15
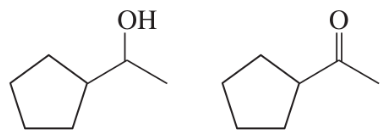
Like nitrogen and carbon, oxygen also shows this same hybridization effect on acidity. Both of the following compounds can lose a proton from a positively charged oxygen with three bonds to give a conjugate base containing a neutral oxygen with two bonds. One of these structures has pKa = −2.4, while the other has pKa = −8.0.
a. Show the reaction of each compound with water.
b. Match each structure with its pKa, and explain your choice.
Problem 16
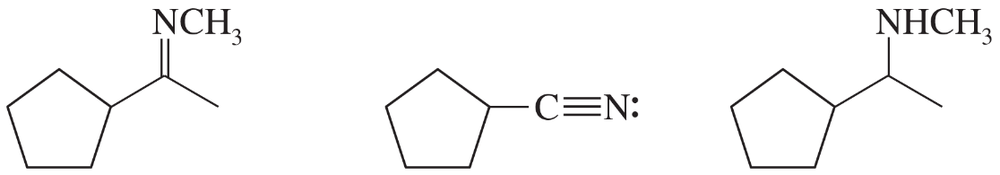
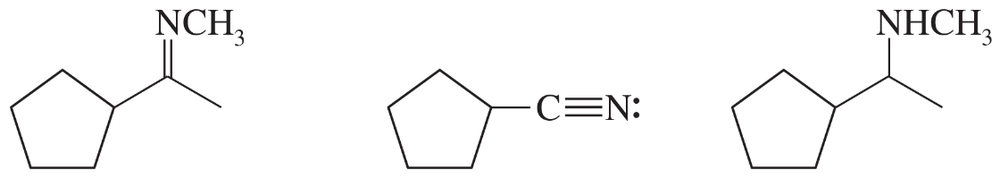
Consider the type of orbitals involved, and rank the following nitrogen compounds in order of decreasing basicity. 2. Rank the conjugate acids in order of increasing acidity. (Hint: These two orders should be the same!)
Problem 16b
Give the structures of their conjugate acids, and estimate their pKas from similar compounds in Appendix 4.
Problem 17a,b
Consider each pair of bases, and explain which one is more basic. Draw their conjugate acids, and show which one is a stronger acid.
(a)
(b)
Problem 18
Which is a stronger base: ethoxide ion or acetate ion? Give pKb values (without looking them up) to support your choice.
Problem 20a
Acetic acid can also react as a very weak base (pKb = 20). Two different sites on acetic acid might become protonated to give the conjugate acid. Draw both of these possible conjugate acids, and explain (resonance) why the correct one is more stable. Calculate the pKa of this conjugate acid.
Problem 21a,b,c
Choose the more acidic member of each pair of isomers, and show why the acid you chose is more acidic.
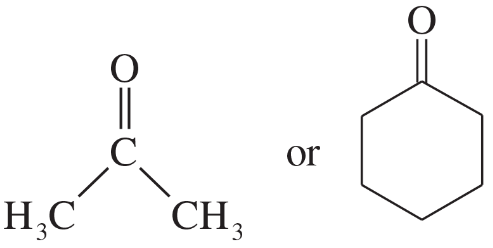
(a)
(b)
(c)