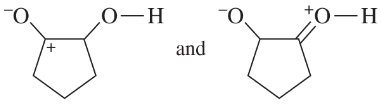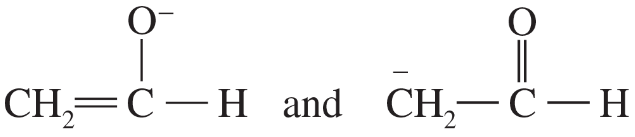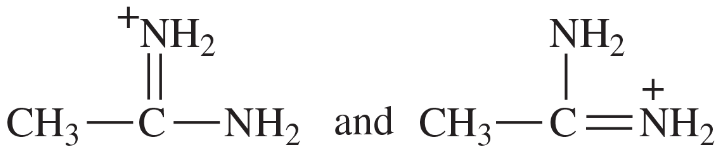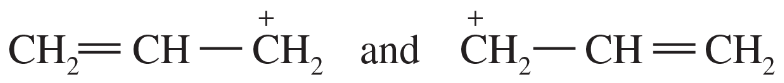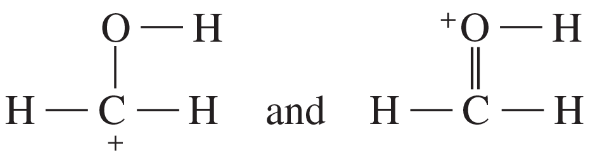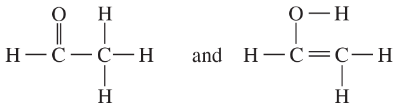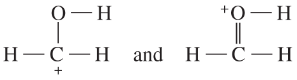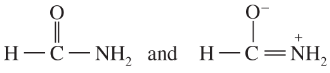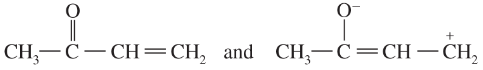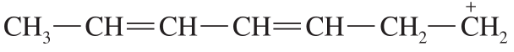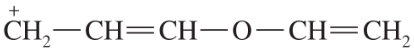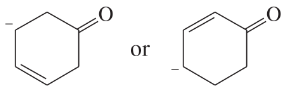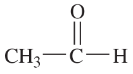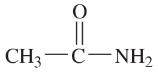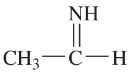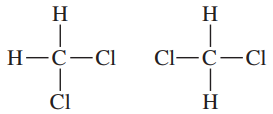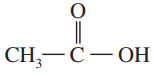Back
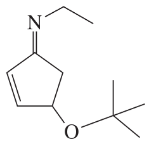
BackProblem 37g,h
Draw complete Lewis structures, including lone pairs, for the following compounds.
(g)
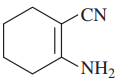
(h)
Problem 38a,b,c
Give the molecular formula of each compound shown
(a)
(b)
(c)
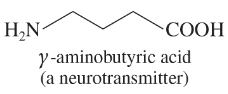
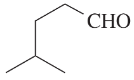
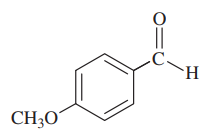
Problem 38d,e,f
Give the molecular formula of each compound shown
(d)
(e)
(f)
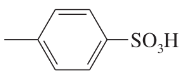
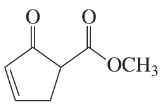
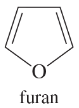
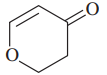
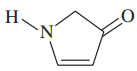
Problem 38g,h
Give the molecular formula of each compound shown
(g)
(h)
Problem 39a-e
1. From what you remember of electronegativities, show the direction of the dipole moments of the following bonds.
2. In each case, predict whether the dipole moment is relatively large (electronegativity difference >0.5) or small.
a. C—Cl
b. C—H
c. C—Li
d. C—N
e. C—O
Problem 40d,e,f
For each of the following structures,
1. Draw a Lewis structure; fill in any nonbonding electrons.
2. Calculate the formal charge on each atom other than hydrogen
d. [(CH3)3O]+
e. CH3NC
f. (CH3)4NBr
Problem 41a-d
Determine whether the following pairs of structures are actually different compounds or simply resonance forms of the same compounds.
a.
b.
c.
d.
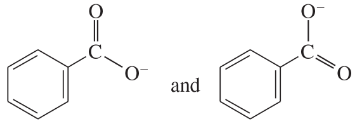
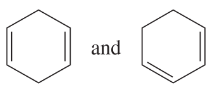
Problem 41e-h
Determine whether the following pairs of structures are actually different compounds or simply resonance forms of the same compounds.
e.
f.
g.
h.
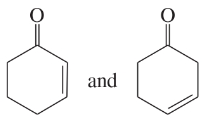
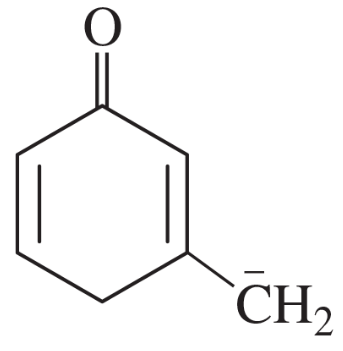
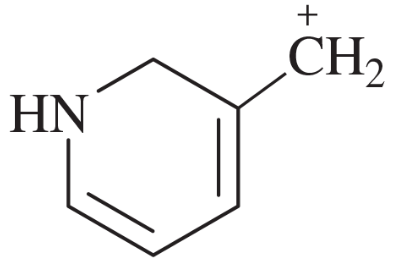
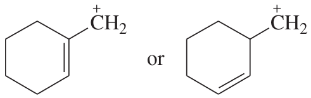
Problem 41i-l
Determine whether the following pairs of structures are actually different compounds or simply resonance forms of the same compounds.
i.
j.
k.
l.
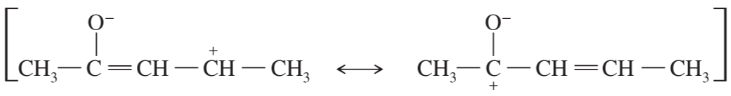
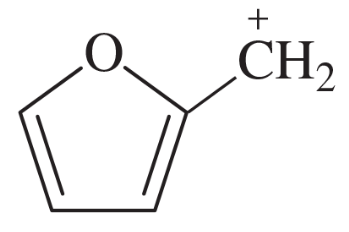
Problem 42j,k
Draw the important resonance forms to show the delocalization of charges in the following ions. In each case, indicate the major resonance form(s).
(j)
(k)
Problem 43d,e
In the following sets of resonance forms, label the major and minor contributors and state which structures would be of equal energy. Add any missing important resonance forms.
(d)
(e)
Problem 44a
For each of these ions, draw the important resonance forms and predict which resonance form is likely to be the major contributor.
(a)
Problem 44b
For each of these ions, draw the important resonance forms and predict which resonance form is likely to be the major contributor.
(b)
Problem 44c
For each of these ions, draw the important resonance forms and predict which resonance form is likely to be the major contributor.
(c)
Problem 45e,f
For each pair of ions, determine which ion is more stable. Use resonance forms to explain your answers.
(e)
(f)
Problem 46a,b,c
Use resonance structures to identify the areas of high and low electron density in the following compounds:
(a)
(b)
(c)
Problem 46g,h
Use resonance structures to identify the areas of high and low electron density in the following compounds:
g.
h.
Problem 46i,j
Use resonance structures to identify the areas of high and low electron density in the following compounds:
i.
j.
Problem 47a
Compound X, isolated from lanolin (sheep’s wool fat), has the pungent aroma of dirty sweatsocks. A careful analysis showed that compound X contains 62.0% carbon and 10.4% hydrogen. No nitrogen or halogen was found.
a. Compute an empirical formula for compound X
Problem 47b
Compound X, isolated from lanolin (sheep's wool fat), has the pungent aroma of dirty sweatsocks. A careful analysis showed that compound X contains 62.0% carbon and 10.4% hydrogen. No nitrogen or halogen was found.
b. A molecular weight determination showed that compound X has a molecular weight of approximately 117. Find the molecular formula of compound X.
Problem 48a
In 1934, Edward A. Doisy of Washington University extracted 3000 lb of hog ovaries to isolate a few milligrams of pure estradiol, a potent female hormone. Doisy burned 5.00 mg of this precious sample in oxygen and found that 14.54 mg of CO2 and 3.97 mg of H2O were generated.
a. Determine the empirical formula of estradiol.
Problem 48b
In 1934, Edward A. Doisy of Washington University extracted 3000 lb of hog ovaries to isolate a few milligrams of pure estradiol, a potent female hormone. Doisy burned 5.00 mg of this precious sample in oxygen and found that 14.54 mg of CO2 and 3.97 mg of H2O were generated.
b. The molecular weight of estradiol was later determined to be 272. Determine the molecular formula of estradiol.
Problem 49
If the carbon atom in CH2Cl2 were flat, there would be two stereoisomers. The carbon atom in CH2Cl2 is actually tetrahedral. Make a model of this compound, and determine whether there are any stereoisomers of CH2Cl2.
Problem 50a,b
Cyclopropane (C3H6, a three-membered ring) is more reactive than most other cycloalkanes.
a. Draw a Lewis structure for cyclopropane.
b. Compare the bond angles of the carbon atoms in cyclopropane with those in an acyclic (noncyclic) alkane.
Problem 50c
Cyclopropane (C3H6, a three-membered ring) is more reactive than most other cycloalkanes.
c. Suggest why cyclopropane is so reactive.
Problem 52a
For each of the following compounds and ions,
1. Draw a Lewis structure.
2. Show the kinds of orbitals that overlap to form each bond.
3. Give approximate bond angles around each atom except hydrogen.
a. [NH2]–
Problem 52b
For each of the following compounds and ions,
1. Draw a Lewis structure.
2. Show the kinds of orbitals that overlap to form each bond.
3. Give approximate bond angles around each atom except hydrogen.
b. [CH2OH]+
Problem 52c
For each of the following compounds and ions,
1. Draw a Lewis structure.
2. Show the kinds of orbitals that overlap to form each bond.
3. Give approximate bond angles around each atom except hydrogen.
c. CH2=N–CH3
Problem 52d,e,f
For each of the following compounds and ions,
1. Draw a Lewis structure.
2. Show the kinds of orbitals that overlap to form each bond.
3. Give approximate bond angles around each atom except hydrogen.
d. CH3–CH=CH2
e. HC≡C–CHO
f. H2N–CH2–CN
Problem 52g
For each of the following compounds and ions,
1. Draw a Lewis structure.
2. Show the kinds of orbitals that overlap to form each bond.
3. Give approximate bond angles around each atom except hydrogen.
g.