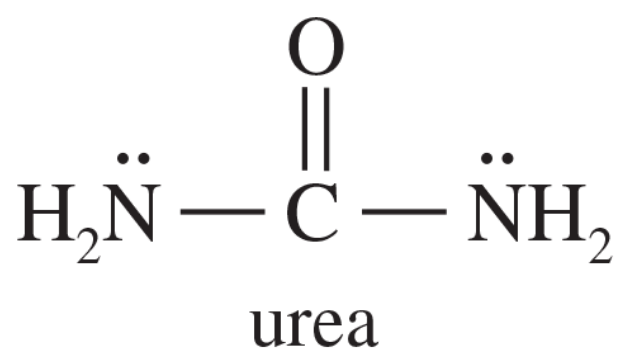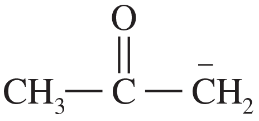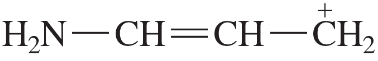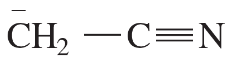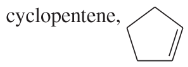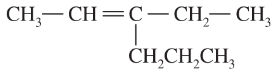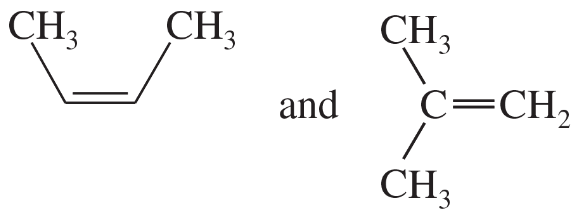Back
BackProblem 52h
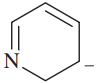
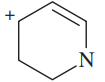
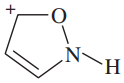
For each of the following compounds and ions,
1. Draw a Lewis structure.
2. Show the kinds of orbitals that overlap to form each bond.
3. Give approximate bond angles around each atom except hydrogen
h.
Problem 53
In most amines, the nitrogen atom is sp3 hybridized, with a pyramidal structure and bond angles close to 109°. In urea, both nitrogen atoms are found to be planar, with bond angles close to 120°. Explain this surprising finding. (Hint: Consider resonance forms and the overlap needed in them.)
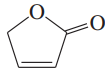
Problem 54a,b,c
Predict the hybridization and geometry of the carbon and nitrogen atoms in the following molecules and ions. (Hint: Resonance.)
a.
b.
c.
Problem 54d
Predict the hybridization and geometry of the carbon and nitrogen atoms in the following molecules and ions. (Hint: Resonance.)
d.
Problem 54e
Predict the hybridization and geometry of the carbon and nitrogen atoms in the following molecules and ions. (Hint: Resonance.)
e.
Problem 54f
Predict the hybridization and geometry of the carbon and nitrogen atoms in the following molecules and ions. (Hint: Resonance.)
f.
Problem 55a,b
Draw orbital pictures of the pi bonding in the following compounds:
a. CH3COCH3
b. HCN
Problem 55e
Draw orbital pictures of the pi bonding in the following compounds:
e. CH3CH=C=CHCH3
Problem 55f
Draw orbital pictures of the pi bonding in the following compounds:
f. CH3CH=NCH=C=O
Problem 56a,b
a. Draw the structure of cis-CH3CH=CHCH2CH3 showing the pi bond with its proper geometry.
b. Circle the six coplanar atoms in this compound.
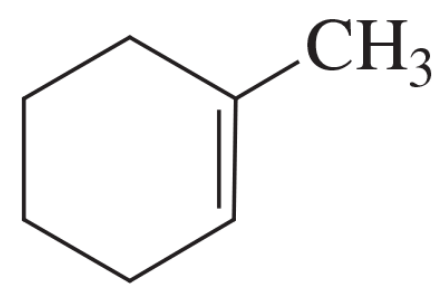
Problem 56d
Circle the coplanar atoms in the following structure:
Problem 57
In pent-2-yne (CH3CCCH2CH3), there are four atoms in a straight line. Use dashed lines and wedges to draw a three-dimensional representation of this molecule, and circle the four atoms that are in a straight line.
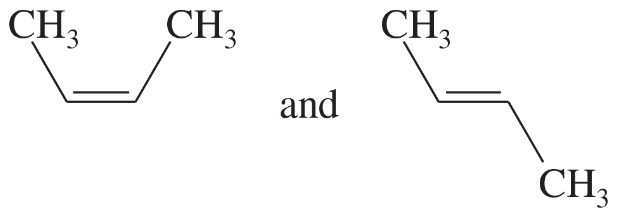
Problem 58a,b,c
Which of the following compounds show cis-trans isomerism? Draw the cis and trans isomers of the ones that do.
(a) CH3CH=CHCH3
(b) CH3C≡CCH3
(c) CH2=C(CH3)2
Problem 58d,e,f
Which of the following compounds show cis-trans isomerism? Draw the cis and trans isomers of the ones that do.
(d)
(e)
(f) CH3CH=NCH3
Problem 59a,b
Give the relationships between the following pairs of structures. The possible relationships are as follows: same compound, cis-trans isomers, constitutional (structural) isomers, and not isomers (different molecular formula).
(a) CH3CH2CH2CH3 and (CH3)3CH
(b) CH2=CH–CH2Cl and CHCl=CH–CH3
Problem 59c,d
Give the relationships between the following pairs of structures. The possible relationships are as follows: same compound, cis-trans isomers, constitutional (structural) isomers, and not isomers (different molecular formula).
(c)
(d)
Problem 60
Dimethyl sulfoxide (DMSO) has been used as an anti-inflammatory rub for race horses. DMSO and acetone appear to have similar structures, but the C=O carbon atom in acetone is planar, while the S=O sulfur atom in DMSO is pyramidal. Draw Lewis structures for DMSO and acetone, predict the hybridizations, and explain these observations.