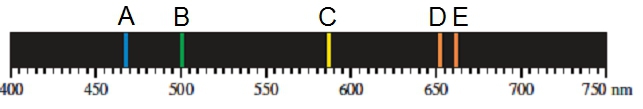
Emission spectra are created when emitted light from an atom is focused through a slit and then passed through a prism. In an atom, electrons occupy various energy levels or shells, theoretically infinite in number. When an electron transitions from a higher energy state to a lower one, it releases energy in the form of light. This process is crucial for understanding how light interacts with matter.
As the electron drops from a higher shell to a lower shell, the emitted energy is focused by a narrow slit, which serves to spread closely packed wavelengths. The light then passes through a prism, which separates the emitted light into its constituent wavelengths, resulting in a series of colored lines known as the emission spectrum. Each line corresponds to a specific wavelength of light, which can be measured and analyzed.
The prism plays a vital role in transforming the emitted wavelengths into discrete lines on the emission spectrum. By examining these lines, we can calculate the wavelengths, and subsequently, the energy and frequency of the emitted light using the relationship:
\[E = h \cdot f\]
where \(E\) is the energy of the photon, \(h\) is Planck's constant (\(6.626 \times 10^{-34} \, \text{Js}\)), and \(f\) is the frequency of the light. This understanding of emission spectra is essential for identifying elements and their properties, as each element has a unique emission spectrum that acts like a fingerprint.