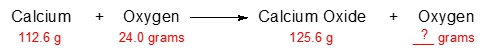The law of conservation of mass, established by the French chemist Antoine Lavoisier, is a fundamental principle in chemistry stating that in a chemical reaction, matter is neither created nor destroyed; it merely changes form. This means that the total mass of the reactants, which are the substances present before the reaction (for example, hydrogen gas \( \text{H}_2 \) and oxygen gas \( \text{O}_2 \)), will equal the total mass of the products formed after the reaction (in this case, water \( \text{H}_2\text{O} \)).
To illustrate this concept, consider a scenario where the combined mass of the reactants is 100 grams. According to the law of conservation of mass, this entire mass will be transformed into products, resulting in exactly 100 grams of water at the end of the reaction. Thus, if you start with a specific mass of reactants, you can predict that the mass of the products will be the same, reinforcing the idea that mass is conserved throughout the chemical process.
This principle is crucial as it lays the groundwork for further topics in chemistry, such as stoichiometry, which involves calculating the quantities of reactants and products in chemical reactions, and solution chemistry, where the conservation of mass helps in understanding concentrations and reactions in solutions. Remember, the law of conservation of mass is essential for determining the potential yield of products in any chemical reaction.