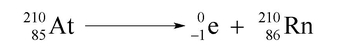
Beta decay is a type of radioactive decay that occurs when an unstable atomic nucleus emits a beta particle, which is essentially an electron. In this process, the beta particle can be represented by the symbol \( e^- \), indicating that it has a negligible atomic mass (effectively 0) and an atomic number of -1. This negative atomic number reflects the fact that an electron is the antiparticle of a proton, which has an atomic number of +1.
To illustrate beta decay, consider the example of mercury-201 (\( \text{Hg}_{80}^{201} \)). In this case, mercury has an atomic number of 80, meaning it contains 80 protons. When mercury-201 undergoes beta decay, it emits a beta particle. The conservation of atomic mass and atomic number must be maintained during this process. Since the emitted electron has no mass, the atomic mass of the resulting element remains 201. However, the atomic number changes due to the emission of the electron.
To determine the new atomic number after the emission, we can set up the equation: \( 80 + (-1) = 81 \). This means that the new element formed after the beta decay will have an atomic number of 81, which corresponds to thallium (Tl). Therefore, the complete beta decay reaction can be represented as:
\[ \text{Hg}_{80}^{201} \rightarrow \text{Tl}_{81}^{201} + e^- \]
This example highlights the key aspects of beta decay, including the emission of a beta particle and the resulting change in atomic number while maintaining the atomic mass.