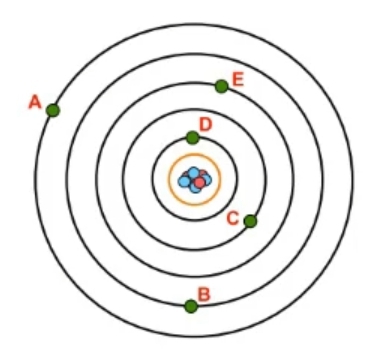
In atomic structure, the concept of shells, denoted by the variable n, represents the grouping of electrons surrounding the nucleus. These shells are crucial as they relate to the potential energy of the electrons, indicating their energy of position. As the value of n increases, both the size and energy level of an atomic orbital also increase. This relationship is directly tied to the periods or rows of the periodic table, where each period corresponds to a specific shell of an atom.
For instance, an atom with seven shells aligns with the seven rows currently present in the periodic table. Each shell corresponds to a period, starting from shell 1 for the first row, progressing through to shell 7 for the seventh row. It is important to note that the periodic table is dynamic; many elements in the seventh row have been discovered or named in recent years. As scientific exploration advances, particularly with new technologies and discoveries on Earth and beyond, the potential for new elements to be created or found is vast. This could lead to the eventual addition of an eighth row, ninth row, and so forth.
The integer value of n must always be a whole number, starting from 1 and theoretically extending to infinity. This means that there is no upper limit to the number of shells an atom can possess, as we are only constrained by our resources and creativity. Thus, the relationship between the rows of the periodic table and the shell numbers of an atom is fundamental, with the understanding that the possible values for n can range from 1 to infinity.