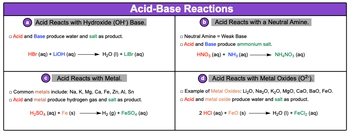
Acid-base reactions are fundamental chemical processes that involve the interaction of acids with various substances, leading to the formation of different products. One common type of reaction occurs when an acid reacts with a hydroxide base, represented by the hydroxide ion (OH-). In this case, the acid and base combine to produce water (H2O) and a salt, which is an ionic compound. For example, when hydrochloric acid (HCl) reacts with lithium hydroxide (LiOH), the hydrogen ion (H+) from the acid combines with the hydroxide ion to form water, while the lithium ion (Li+) and chloride ion (Cl-) combine to form lithium chloride (LiCl) in aqueous solution.
Another type of acid-base reaction involves neutral amines, which are weak bases containing nitrogen and hydrogen, or carbon, nitrogen, and hydrogen. For instance, ammonia (NH3) and methylamine are examples of neutral amines. When an acid, such as nitric acid (HNO3), reacts with ammonia, the products formed include ammonium nitrate (NH4NO3), a salt that is also an ionic compound.
Acids can also react with metals, producing hydrogen gas (H2) and a salt. Common metals that participate in these reactions include sodium, potassium, magnesium, calcium, iron, zinc, aluminum, and tin. For example, when sulfuric acid (H2SO4) reacts with iron (Fe), hydrogen gas is released, and iron(II) sulfate (FeSO4) is formed as the salt.
Lastly, acids can react with metal oxides, such as lithium oxide (Li2O) or iron(II) oxide (FeO). In these reactions, the products are water and a salt. For instance, when hydrochloric acid reacts with iron(II) oxide, water is produced along with iron(II) chloride (FeCl2).
Understanding these patterns of acid-base reactions is crucial for predicting the products formed based on the type of acid and the reacting substance. Recognizing the nature of the compounds involved allows for effective identification of the resulting products in these chemical processes.