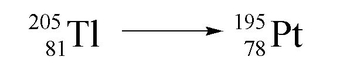
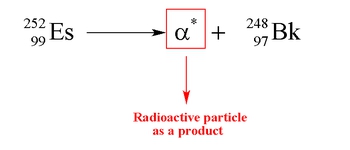
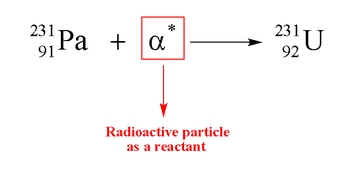
Nuclear reactions involve processes that occur within unstable atomic nuclei, fundamentally altering the number of protons in an atom. In a typical atom, electrons orbit a nucleus composed of protons, which carry a positive charge, and neutrons, which are neutral. The atomic number, defined by the number of protons, determines the identity of an element. Unlike conventional chemical reactions, where the identities of the reactants remain unchanged, nuclear reactions can transform one element into another entirely different element.
For instance, in a chemical reaction, we might see a balanced equation such as:
H_2 + N_2 \rightarrow NH_3
Here, the identities of hydrogen and nitrogen remain the same throughout the reaction. However, in nuclear reactions, the manipulation of protons can lead to the formation of new elements. For example, a nucleus of calcium-40 can undergo a transformation that results in the creation of argon. This illustrates that by changing the number of protons, we directly influence the identity of the element, highlighting the unique nature of nuclear chemistry.