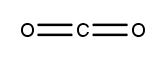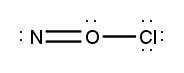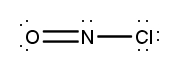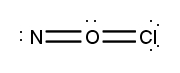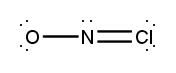Atoms often form multiple bonds when their valence electrons are insufficient to satisfy the octet rule, which states that atoms prefer to have eight electrons in their outer shell, similar to the electron configuration of noble gases. For instance, consider nitrogen, which can form either a single bond or a triple bond with another nitrogen atom. In the case of a single bond, a total of 10 valence electrons are utilized, as each bond consists of 2 electrons. However, this configuration leaves both nitrogen atoms with only 6 electrons each, failing to meet the octet requirement.
To resolve this, forming a triple bond is more effective. In a triple bond, the total number of valence electrons remains 10, but each nitrogen atom now shares three pairs of electrons, resulting in 8 electrons around each atom. This arrangement satisfies the octet rule, demonstrating that multiple bonds, such as double or triple bonds, may be necessary to achieve a complete octet for certain elements.
It is important to note that hydrogen is an exception to the octet rule; it adheres to the duet rule, desiring only 2 electrons in its outer shell to achieve a stable configuration akin to helium.