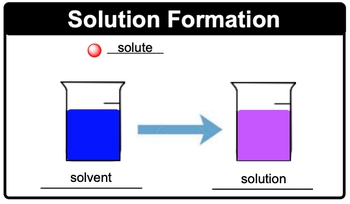
Solutions are defined as homogeneous mixtures composed of two or more components that blend to form a uniform composition. This uniformity means that, upon observation, it is impossible to distinguish the individual components, as they mix perfectly together. Within a solution, there are two key components to understand: the solute and the solvent.
The solute is the smaller portion of the solution that gets dissolved, while the solvent is the component present in a larger amount, capable of dissolving other substances. For example, if we consider a solution where the solute is a small ball and the solvent is represented by a larger body of water, the solute will dissolve in the solvent, resulting in a new mixture, often depicted as a colored solution.
Another important concept related to solutions is concentration, which refers to the measurement of the amount of solute present in a given volume of solution. Understanding concentration is crucial for various applications in chemistry, as it helps quantify how much solute is dissolved in the solvent.
In summary, solutions are homogeneous mixtures formed by the dissolution of a solute in a solvent, resulting in a uniform composition that is essential for many chemical processes.