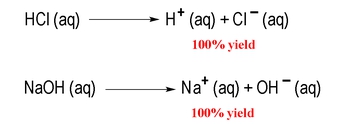
Understanding the relationship between pH and pOH is crucial when studying strong acids and strong bases, which are classified as strong electrolytes. Strong electrolytes are substances that completely ionize in solution, meaning they dissociate 100% into their constituent ions. For instance, hydrochloric acid (HCl) is a strong binary acid, while sodium hydroxide (NaOH) is a strong base, characterized by the presence of a group 1A ion (Na+) paired with hydroxide (OH-).
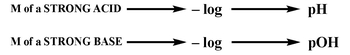
When a strong acid or base is dissolved in water, it fully dissociates, leading to a reaction that can be represented with a single arrow pointing forward, indicating that the products are highly favored. This complete ionization simplifies the calculation of pH and pOH. For strong acids, the pH can be determined using the formula:
pH = -\log[H^+]
Similarly, for strong bases, pOH is calculated as:
pOH = -\log[OH^-]
These calculations eliminate the need for an ICE (Initial, Change, Equilibrium) chart, streamlining the process of determining the acidity or basicity of a solution. In the following example, we will apply these principles to calculate pH and pOH for specific strong acid and base scenarios.