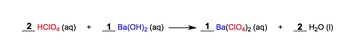
A molecular equation represents the intact compounds involved in a chemical reaction, rather than their dissociated ionic forms. In a typical molecular equation, two aqueous reactants combine to yield products, which can also be in aqueous form or other states. For instance, when 2 moles of perchloric acid (HClO₄) react with 1 mole of barium hydroxide (Ba(OH)₂), the products formed are 1 mole of barium perchlorate (Ba(ClO₄)₂) and 2 moles of water (H₂O).
There are various types of molecular equations based on the products formed. In a neutralization reaction, an aqueous acid reacts with an aqueous base, resulting in the formation of an ionic compound and water. This is a fundamental type of acid-base reaction where the products typically include water and a salt.
In contrast, gas evolution reactions involve the production of a gas as one of the products. Here, two aqueous reactants react to produce at least one gaseous product, which may be accompanied by another product that could also be a gas, but this is not guaranteed.
Lastly, precipitation reactions are characterized by the formation of a solid, known as a precipitate, from the reaction of two aqueous reactants. This solid ionic compound forms when the solubility rules indicate that at least one of the products is insoluble in water.
In summary, molecular equations illustrate the interaction of aqueous reactants to form various products, which can be categorized into neutralization, gas evolution, and precipitation reactions based on the nature of the products formed.