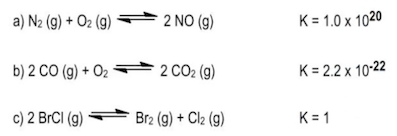
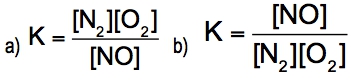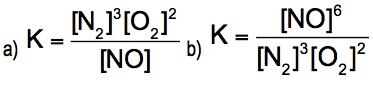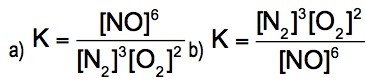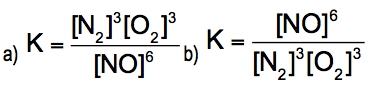The equilibrium constant, denoted as \( K \), is a crucial concept in chemical equilibrium, representing the ratio of the concentrations of products to reactants at a specific temperature. This relationship can be expressed mathematically as:
\[ K = \frac{[\text{Products}]}{[\text{Reactants}]} \]
Temperature plays a significant role in determining the value of \( K \); an increase in temperature generally leads to an increase in \( K \), while a decrease in temperature results in a lower \( K \). The magnitude of \( K \) provides insight into the position of equilibrium in a chemical reaction. When \( K > 1 \), it indicates that products are favored over reactants, suggesting that the reaction proceeds predominantly in the forward direction. For example, if the concentration of products is 10 and that of reactants is 1, then:
\[ K = \frac{10}{1} = 10 \]
This value, being greater than 1, confirms that the formation of products is favored. Conversely, if \( K < 1 \), such as when products are 1 and reactants are 10, the equilibrium shifts towards the reactants, indicating a preference for the reverse reaction:
\[ K = \frac{1}{10} = 0.1 \]
In this case, the reaction favors the formation of reactants. When \( K = 1 \), it signifies that the concentrations of products and reactants are equal, indicating a balanced state:
\[ K = \frac{10}{10} = 1 \]
It is important to note that the equilibrium constant \( K \) considers all states of matter in a reaction, except for solids and pure liquids, which are not included in the expression for \( K \). This exclusion is due to their constant concentrations during the reaction, which do not affect the equilibrium position.