Open Question
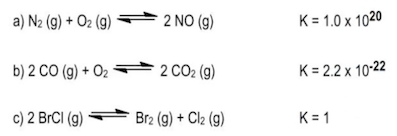
Use your answer from Problem 7.54 to calculate the following:[O2] at equilibrium when [CO2] = 0.18 mol/L and [CO] = 0.0200 mol/L
 Verified step by step guidance
Verified step by step guidance
 2:50m
2:50mMaster The Equilibrium Constant Concept 1 with a bite sized video explanation from Jules
Start learning
