Open Question
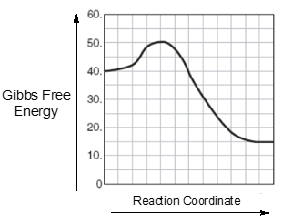
Which reaction is faster, one with E_act = +10 kcal/mol(+41.8 kJ/mol) or one with E_act = +5 kcal/mol(+20.9 kJ/mol)? Explain.
 Verified step by step guidance
Verified step by step guidance
 2:40m
2:40mMaster Energy Diagrams Concept 1 with a bite sized video explanation from Jules
Start learning
