Textbook Question
What kind of biological function would each of the following proteins perform?
c. Protease

 Verified step by step guidance
Verified step by step guidance

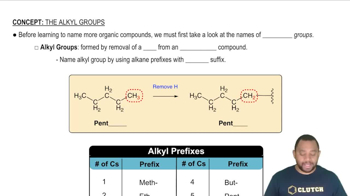
What kind of biological function would each of the following proteins perform?
c. Protease
What amino acids do the following abbreviations stand for? Draw the structure of each.
a. Val
Name and draw the structures of the amino acids that fit the following descriptions:
a. Contains a thiol group
Draw leucine and identify any chiral carbon atoms with arrows.
Is phenylalanine hydrophilic or hydrophobic? Explain why.
At neutral pH, which of the following amino acids has a net positive charge, which has a net negative charge, and which is neutral? (Hint: Draw the various charged forms of each amino acid before deciding.)
a. Asparagine
b. Lysine
c. Proline