Bradykinin, a peptide that helps to regulate blood pressure, has the primary structure Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg.
a. Draw the complete structural formula of bradykinin.

 Verified step by step guidance
Verified step by step guidance
Bradykinin, a peptide that helps to regulate blood pressure, has the primary structure Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg.
a. Draw the complete structural formula of bradykinin.
Bradykinin, a peptide that helps to regulate blood pressure, has the primary structure Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg.
b. Bradykinin has a very kinked secondary structure. Why?
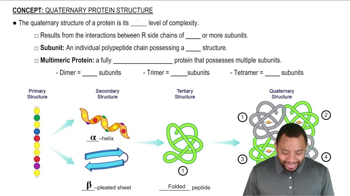
Give an example of a protein containing primarily alpha-helices. Is this a fibrous or globular protein?
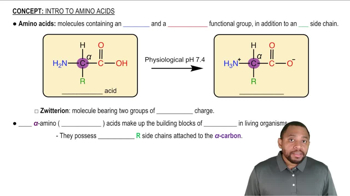
What kind of bond would you expect between the side chains of the following amino acids?
c. Aspartic acid and asparagine
Is the bond formed between each pair in Problem 18.76 covalent or noncovalent?
a. Cysteine and cysteine
b. Alanine and leucine
c. Aspartic acid and asparagine
d. Serine and lysine
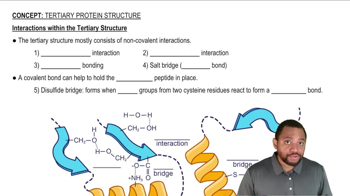
What is meant by the following terms as they apply to protein structure, and what bonds or molecular interactions stabilize that level of structure?
a. Primary structure