Identify the functional groups in cocaine
Ch.16 Amines

Chapter 16, Problem 42a
Complete the following equations. (Hint: Remember that a nitrogen with three groups bound to it has a lone pair and one with four does not)
a. 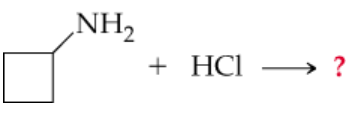
 Verified step by step guidance
Verified step by step guidance1
Identify the type of nitrogen atom in the given molecule. Determine whether it has three groups bound to it (indicating a lone pair is present) or four groups (indicating no lone pair). This will help you predict its reactivity and bonding behavior.
Examine the reactants and products in the equation. Consider the role of nitrogen in the reaction, such as whether it is acting as a nucleophile (donating its lone pair) or forming a new bond.
Apply the principles of valence and bonding. For nitrogen, ensure that it follows the octet rule, unless there are exceptions due to resonance or other factors.
Balance the chemical equation by ensuring that the number of atoms of each element is the same on both sides of the equation. Also, check that the charges are balanced if the reaction involves ions.
Review the completed equation to confirm that it adheres to the rules of chemical reactivity and that the nitrogen atom's bonding and lone pair status are consistent with the hints provided.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Valence Shell Electron Pair Repulsion (VSEPR) Theory
VSEPR theory is a model used to predict the geometry of individual molecules based on the repulsion between electron pairs in the valence shell of the central atom. According to this theory, electron pairs will arrange themselves as far apart as possible to minimize repulsion, which helps determine the molecular shape.
Recommended video:
Guided course

Valence Shell Electron Pair Repulsion Theory (Simplified) Concept 1
Hybridization
Hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals that can accommodate the bonding requirements of a molecule. For example, sp3 hybridization occurs when one s orbital and three p orbitals mix to form four equivalent sp3 hybrid orbitals, which is common in tetrahedral geometries.
Recommended video:
Guided course
Resonance Structures (Simplified) Concept 1
Lone Pairs
Lone pairs are pairs of valence electrons that are not involved in bonding and are localized on a single atom. The presence of lone pairs can significantly influence the geometry of a molecule, as they occupy space and can repel bonding pairs, altering bond angles and overall molecular shape.
Recommended video:
Guided course
Base Pairing Concept 1
Related Practice
Textbook Question
Textbook Question
Draw the structures of the ammonium ions formed when the amines in Problem 16.30 are treated with acid.
a. N-Methylpentylamine
b. N-Ethylcyclobutylamine
c. p-Propylaniline
Textbook Question
Complete the following equations (hint: remember that a nitrogen with three groups bound to it has a lone pair and one with four does not):
a.
Textbook Question
Many hair conditioners contain an ammonium salt such as the following to help prevent 'fly-away' hair. These ions will react with neither acid nor base. Provide a reason why.
Textbook Question
Choline has the following structure. Do you think that this substance reacts with aqueous hydrochloric acid? If so, what is the product? If not, why not?
Textbook Question
Which is the stronger base, trimethylamine or ammonia? In which direction will the following reaction proceed?
