Why do aldehydes and ketones have lower boiling points than alcohols with similar molecular weights? Why are their boiling points higher than those of alkanes with similar molecular weights?
Ch.15 Aldehydes and Ketones

Chapter 15, Problem 15.48
Aldosterone is a key steroid involved in controlling the sodium–potassium balance in the body. Identify the functional groups in aldosterone. <IMAGE>
 Verified step by step guidance
Verified step by step guidance1
Identify the structure of aldosterone from the image provided or from a reliable source. Aldosterone is a steroid, so its structure will include a tetracyclic cyclopentanoperhydrophenanthrene nucleus.
Look for the ketone groups in the structure. Ketones are characterized by a carbonyl group (C=O) attached to two carbon atoms. In aldosterone, you will find a ketone group at the C18 position.
Identify any alcohol groups present. Alcohols are characterized by a hydroxyl group (-OH) attached to a carbon atom. Aldosterone has hydroxyl groups at the C11 and C21 positions.
Check for the presence of an aldehyde group. Aldehydes feature a carbonyl group (C=O) attached to at least one hydrogen atom. Aldosterone does not typically have an aldehyde group, but verify this in the structure you are examining.
Examine the molecule for any other functional groups such as double bonds or additional oxygen-containing groups that might be relevant to its chemical properties and biological function.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Functional Groups
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. In organic chemistry, they determine the properties and reactivity of compounds. Common functional groups include hydroxyl, carboxyl, and amino groups, each influencing the behavior of the molecule in biological systems.
Recommended video:
Guided course
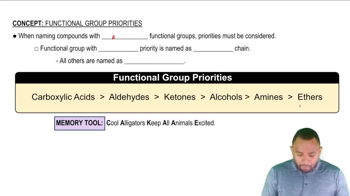
Functional Group Priorities Concept 1
Steroid Structure
Steroids are a class of lipids characterized by a core structure of four fused carbon rings. This unique arrangement allows steroids like aldosterone to interact with specific receptors in the body, influencing various physiological processes, including metabolism and electrolyte balance. Understanding the steroid structure is crucial for identifying its functional groups.
Recommended video:
Guided course
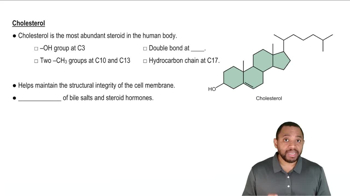
Steroids Concept 2
Aldosterone Function
Aldosterone is a steroid hormone produced by the adrenal glands that plays a vital role in regulating sodium and potassium levels in the body. It promotes sodium reabsorption and potassium excretion in the kidneys, which helps maintain blood pressure and fluid balance. Recognizing its function aids in understanding the significance of its chemical structure and functional groups.
Recommended video:
Guided course

Logarithmic Functions
Related Practice
Textbook Question
Textbook Question
What ketones or aldehydes might be reduced to yield the following alcohols?
a.
b.
c. HOCH2–CH2–CH2OH
Textbook Question
Determine whether the following compounds are acetals or ketals. Draw the structure of the aldehyde or ketone it came from.
c.
Textbook Question
Draw the structures of the hemiacetals or hemiketals formed in these reactions:
b.
Textbook Question
For each compound shown next, determine whether it is a hemiacetal, a hemiketal, an acetal, or a ketal.
a.
b.
c.
d.
Textbook Question
The carbonyl group can be reduced by addition of a hydride ion (H–) and (H+) a proton. Removal of H– and H+ from an alcohol results in a carbonyl group.
a. To which atom of the carbonyl is the hydride ion added and why?
