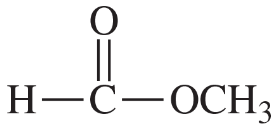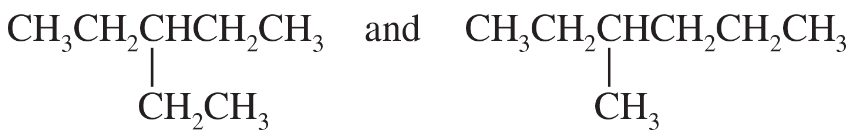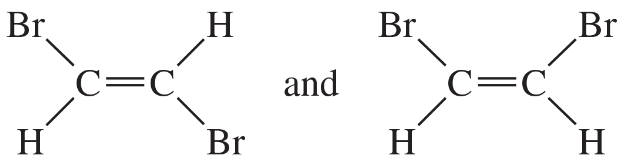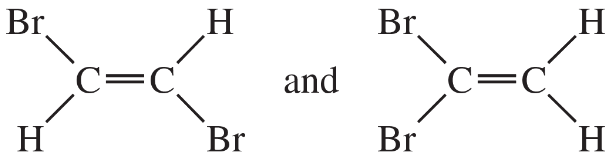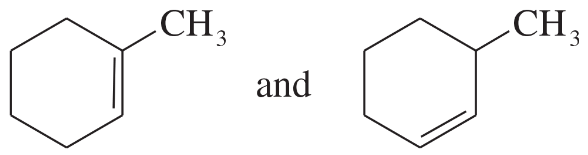Back
BackProblem 10d
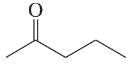
Use resonance structures to identify the areas of high and low electron density in the following compounds:
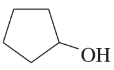
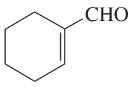
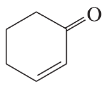
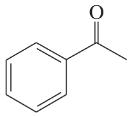
(d)
Problem 11d,e
Draw complete Lewis structures for the following condensed structural formulas.
(d) CH2CHCHO
(e) (CH3)3CCOCHCH2
Problem 12a,b
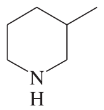
Give Lewis structures corresponding to the following line–angle structures. Give the molecular formula for each structure.
(a)
(b)
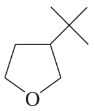
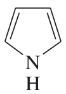
Problem 12c,d
Give Lewis structures corresponding to the following line–angle structures. Give the molecular formula for each structure.
(c)
(d)
Problem 12e,f
Give Lewis structures corresponding to the following line–angle structures. Give the molecular formula for each structure.
(e)
(f)
Problem 12g,h
Give Lewis structures corresponding to the following line–angle structures. Give the molecular formula for each structure.
(g)
(h)
Problem 13a,b
Draw line-angle structures for the compounds (a) through (h).
a. CH3(CH2)3CH(CH3)2
b. (CH3)2CHCH2Cl
Problem 13c,d
Draw line-angle structures for the compounds (a) through (h).
c. CH3CH2COCN
d. CH2CHCHO
Problem 13e,f
Draw line-angle structures for the compounds (a) through (h).
e. (CH3)3CCOCHCH2
f. CH3COCOOH
Problem 13g,h
Draw line-angle structures for the compounds (a) through (h).
g. (CH3CH2)2CO
h. (CH3)3COH
Problem 14a
Compute the empirical and molecular formulas for each of the following elemental analyses. In each case, propose at least one structure that fits the molecular formula.
Problem 14b
Compute the empirical and molecular formulas for each of the following elemental analyses. In each case, propose at least one structure that fits the molecular formula.
Problem 14c
Compute the empirical and molecular formulas for each of the following elemental analyses. In each case, propose at least one structure that fits the molecular formula.
Problem 14d
Compute the empirical and molecular formulas for each of the following elemental analyses. In each case, propose at least one structure that fits the molecular formula.
Problem 15b
Make a model of propane (C3H8), and draw this model using dashed lines and wedges to represent bonds going back and coming forward.
Problem 16a
Predict the hybridization of the oxygen atom in water, H2O. Draw a picture of its three-dimensional structure, and explain why its bond angle is 104.5°.
Problem 16b
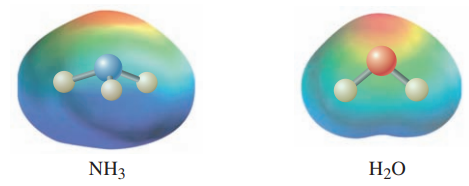
The electrostatic potential maps for ammonia and water are shown here. The structure of ammonia is shown within its EPM. Note how the lone pair creates a region of high electron potential (red), and the hydrogens are in regions of low electron potential (blue). Show how your three-dimensional structure of water corresponds with its EPM.
Problem 17a
Predict the hybridization, geometry, and bond angles for the central atoms in
a. but-2-ene, CH3CH=CHCH3
Problem 17b
Predict the hybridization, geometry, and bond angles for the central atoms in
b. CH3CH=NH
Problem 18
Predict the hybridization, geometry, and bond angles for the carbon and nitrogen atoms in acetonitrile (CH3–C≡N:).
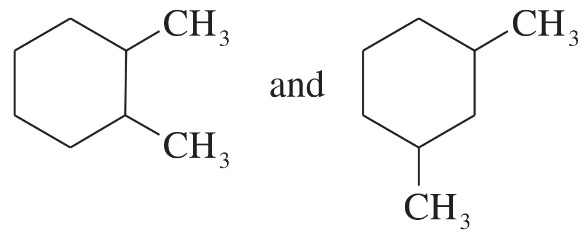
Problem 22
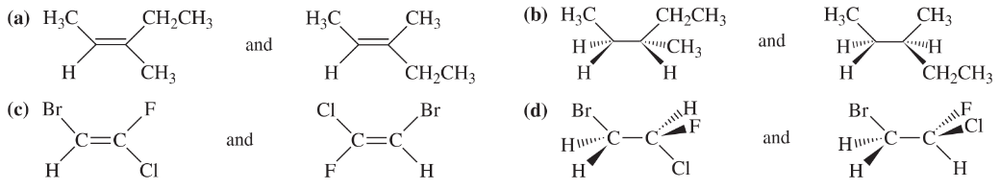
For each pair of structures, determine whether they represent different compounds or a single compound.
Problem 23b,c
Two compounds with the formula CH3–CH=N–CH3 are known.
b. What two compounds have this formula?
c. Explain why only one compound with the formula (CH3)2CNCH3 is known.
Problem 24a,b,c
Which of the following compounds show cis-trans isomerism? Draw the cis and trans isomers of those that do.
(a) CHF=CHF
(b) F2C=CH2
(c) CH2=CH–CH2–CH3
Problem 25a,b,c
Give the relationship between the following pairs of structures. The possible relationships are:
same compound
constitutional isomers (structural isomers)
cis-trans isomers
not isomers (different molecular formula)
(a)
(b)
(c)
Problem 25g,h,i
Give the relationship between the following pairs of structures. The possible relationships are:
same compound
constitutional isomers (structural isomers)
cis-trans isomers
not isomers (different molecular formula)
(g) CH3–CH2–CH2–CH3 and CH3–CH=CH–CH3
(h) CH2=CH–CH2CH2CH3 and CH3–CH=CH–CH2CH3
(i) CH2=CHCH2CH2CH3 and CH3CH2CH2CH=CH2
Problem 25j,k
Give the relationship between the following pairs of structures. The possible relationships are:
same compound
constitutional isomers (structural isomers)
cis-trans isomers
not isomers (different molecular formula)
(j)
(k)
Problem 26a
a. Draw the resonance forms for SO2 (bonded O–S–O).
Problem 26b,c
b. Draw the resonance forms for ozone (bonded O–O–O)
c. Sulfur dioxide has one more resonance form than ozone. Explain why this structure is not possible for ozone.
Problem 27
Name the element that corresponds to each electronic configuration.
a. 1s2 2s2 2p2
b. 1s2 2s2 2p4
c. 1s2 2s2 2p6 3s2 3p3
d. 1s2 2s2 2p6 3s2 3p5
Problem 28
There is a small portion of the periodic table that you must know to do organic chemistry. Construct this part from memory, using the following steps.
a. From memory, make a list of the elements in the first two rows of the periodic table, together with their numbers of valence electrons
b. Use this list to construct the first two rows of the periodic table.
c. Organic compounds often contain sulfur, phosphorus, chlorine, bromine, and iodine. Add these elements to your periodic table.