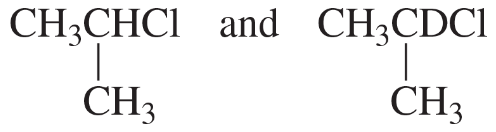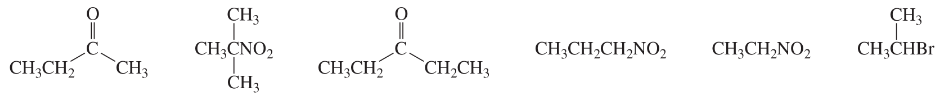Back
BackProblem 50
Determine the ratios of the chemically nonequivalent protons in a compound if the steps of the integration curves measure 40.5, 27, 13, and 118 mm, from left to right across the spectrum. Draw the structure of a compound whose 1H NMR spectrum would show these integrals in the observed order.
Problem 51g
How can 1H NMR distinguish between the compounds in each of the following pairs?
g.
Problem 52c,d
Answer the following questions:
c. What is the relationship between coupling constant in hertz and operating frequency?
d. How does the operating frequency in NMR spectroscopy compare with the operating frequency in IR and UV/Vis spectroscopy?
Problem 53b
Match each of the 1H NMR spectra with one of the following compounds:
b. <IMAGE>
Problem 54a
The 1H NMR spectra of three isomers with molecular formula C4H9Br are shown here. Which isomer produces which spectrum?
a. <IMAGE>
Problem 54b
The 1H NMR spectra of three isomers with molecular formula C4H9Br are shown here. Which isomer produces which spectrum?
b. <IMAGE>
Problem 54c
The 1H NMR spectra of three isomers with molecular formula C4H9Br are shown here. Which isomer produces which spectrum?
c. <IMAGE>
Problem 55b
Identify each of the following compounds from the 1H NMR data and molecular formula:
b. C8H9Br: a 3H doublet at 2.01 ppm a 1H quartet at 5.14 ppm
a 5H broad singlet at 7.35 ppm
Problem 55c
Identify each of the following compounds from the 1H NMR data and molecular formula:
c. C5H10O2: a 3H triplet at 1.15 ppm
a 3H triplet at 1.25 ppm
a 2H quartet at 2.33 ppm
a 2H quartet at 4.13 ppm
Problem 56
Identify the compound with molecular formula C7H14O that gives the following proton-coupled 13C NMR spectrum:
<IMAGE>
Problem 57
Compound A, with molecular formula C4H9Cl, shows two signals in its 13C NMR spectrum. Compound B, an isomer of compound A, shows four signals, and in the proton-coupled mode, the signal farthest downfield is a doublet. Identify compounds A and B.
Problem 58
Would it be better to use 1H NMR or 13C NMR spectroscopy to distinguish 1-butene, cis-2-butene, and 2-methylpropene? Explain your answer.
Problem 61
An alkyl halide reacts with an alkoxide ion to form a compound whose 1H NMR spectrum is shown here. Identify the alkyl halide and the alkoxide ion. (Hint: See Section 9.15.)
<IMAGE>
Problem 62
The 1H NMR spectra of three isomers with molecular formula C7H14O are shown here. Which isomer produces which spectrum?
b. <IMAGE>
Problem 63b
Identify each of the following compounds from its molecular formula and its IR and 1H NMR spectra:
b. C6H12O2
<IMAGE>
Problem 63d
Identify each of the following compounds from its molecular formula and its IR and 1H NMR spectra.
d. C4H8O2
<IMAGE>
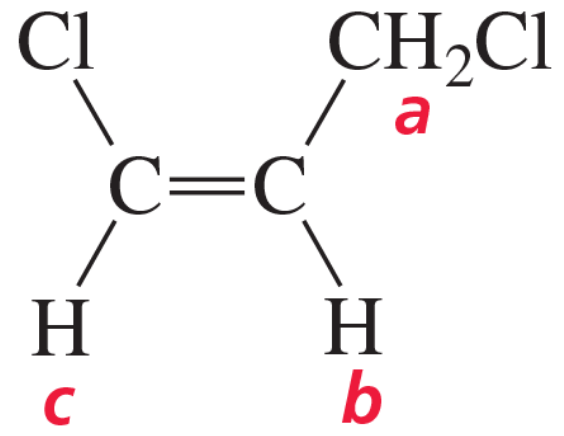
Problem 65
The 1H NMR spectrum of 2-propen-1-ol is shown here. Indicate the protons in the molecule that are responsible for each of the signals in the spectrum.
<IMAGE>
Problem 68
Draw a splitting diagram for the Hb proton if Jbc = 10 and Jba = 5.
Problem 69a
Sketch the following spectra that would be obtained for 2-chloroethanol:
a. The 1H NMR spectrum for an anhydrous sample of the alcohol.
Problem 69b
Sketch the following spectra that would be obtained for 2-chloroethanol:
b. The 1H NMR spectrum for a sample of the alcohol that contains a trace amount of acid.
Problem 69c
Sketch the following spectra that would be obtained for 2-chloroethanol:
c. The 13C NMR spectrum.
Problem 69d
Sketch the following spectra that would be obtained for 2-chloroethanol:
d. The proton-coupled 13C NMR spectrum.
Problem 69e
Sketch the following spectra that would be obtained for 2-chloroethanol:
e. The four parts of a DEPT 13C NMR spectrum.
Problem 70
How can 1H NMR be used to prove that the addition of HBr to propene follows the rule that says that the electrophile adds to the sp2 carbon bonded to the most hydrogens?
Problem 71b
Identify each of the following compounds from its molecular formula and its 1H NMR spectrum:
b. C6H12O
<IMAGE>
Problem 73
Calculate the amount of energy (in calories) required to flip an 1H nucleus in an NMR spectrometer that operates at 300 MHz.
Problem 74c
The following 1H NMR spectra are for four compounds, each with molecular formula of C6H12O2. Identify the compounds.
c. <IMAGE>
Problem 74d
The following 1H NMR spectra are for four compounds, each with molecular formula of C6H12O2. Identify the compounds.
d. <IMAGE>
Problem 75
When compound A (C5H12O) is treated with HBr, it forms compound B (C5H11Br). The 1H NMR spectrum of compound A has a 1H singlet, a 3H doublet, a 6H doublet, and two 1H multiplets. The 1H NMR spectrum of compound B has a 6H singlet, a 3H triplet, and a 2H quartet. Identify compounds A and B.
Problem 76
Identify the compound with molecular formula C6H10O that gives the following DEPT 13C NMR spectrum:
<IMAGE>