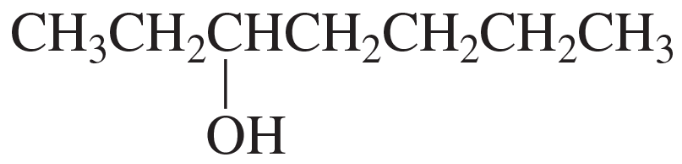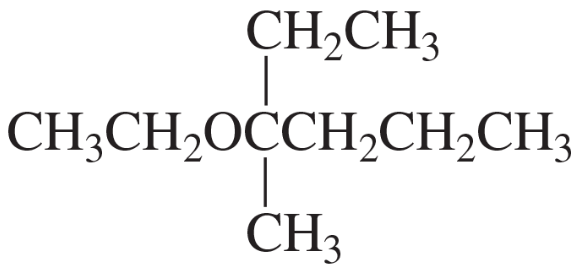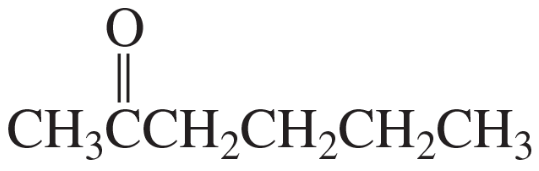Back
Back Bruice 8th Edition
Bruice 8th Edition Ch. 13 - Mass Spectrometry; Infrared Spectroscopy; UV/Vis Spectroscopy
Ch. 13 - Mass Spectrometry; Infrared Spectroscopy; UV/Vis SpectroscopyProblem 2
What distinguishes the mass spectrum of 2,2-dimethylpropane from the mass spectra of pentane and isopentane?
Problem 3
What is the most likely m/z value for the base peak in the mass spectrum of 3-methylpentane?
Problem 5b
Determine the molecular formula for each of the following:
b. a compound that contains C, H, and one O and has a molecular ion with an m/z value of 100
Problem 6a
Suggest possible molecular formulas for a compound that has a molecular ion with an m/z value of 86.
Problem 6b
Can one of the possible molecular formulas contain a nitrogen atom?
Problem 8
Identify the hydrocarbon that has a molecular ion with an m/z value of 128, a base peak with an m/z value of 43, and significant peaks with m/z values of 57, 71, and 85.
Problem 9
Predict the relative intensities of the molecular ion peak, the M+2 peak, and the M+4 peak for a compound that contains two bromine atoms.
Problem 11
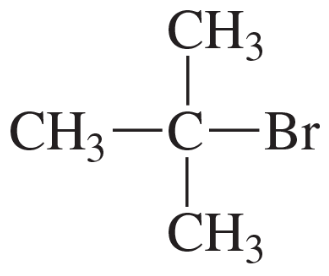
Can a high-resolution mass spectrometer distinguish between them?
Problem 13a
The mass spectra of 1-methoxybutane, 2-methoxybutane, and 2-methoxy-2-methylpropane are shown below. Match each compound with its spectrum.
<IMAGE>
Problem 14a
Primary alcohols have a strong peak at m/z = 31.
a. What fragment is responsible for this peak?
Problem 17b
Using curved arrows, show the principal fragments you would expect to see in the mass spectrum of each of the following compounds:
b.
Problem 17c
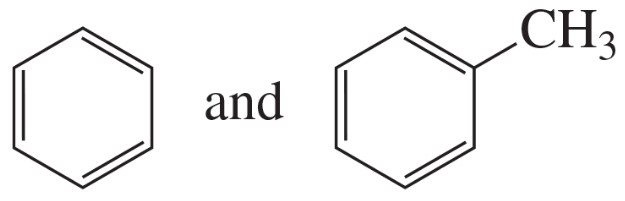
Using curved arrows, show the principal fragments you would expect to see in the mass spectrum of each of the following compounds:
c.
Problem 17d
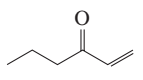
Using curved arrows, show the principal fragments you would expect to see in the mass spectrum of each of the following compounds:
d.
Problem 17f
Using curved arrows, show the principal fragments you would expect to see in the mass spectrum of each of the following compounds:
f.
Problem 18
The reaction of (Z)-2-pentene with water and a trace of H2SO4 forms two products. Identify the products from their mass spectra.
<IMAGE>
Problem 21a(3,4)
a. Which occurs at a larger wavenumber:
3. a C–N stretch or a C=N stretch?
4. a C=O stretch or a C–O stretch?
Problem 21a(1,2)
a. Which occurs at a larger wavenumber:
1. a C=C stretch or a C=C stretch?
2. a C–H stretch or a C–H bend?
Problem 22
Which occurs at a larger wavenumber:
a. the C–O stretch of phenol or the C–O stretch of cyclohexanol?
b. the C=O stretch of a ketone or the C=O stretch of an amide?
c. the C–N stretch of cyclohexylamine or the C–N stretch of aniline?
Problem 24
Why is the C–O absorption band of 1-hexanol at a smaller wavenumber (1060 cm–1) than the C–O absorption band of pentanoic acid (1220 cm–1)?
Problem 25b
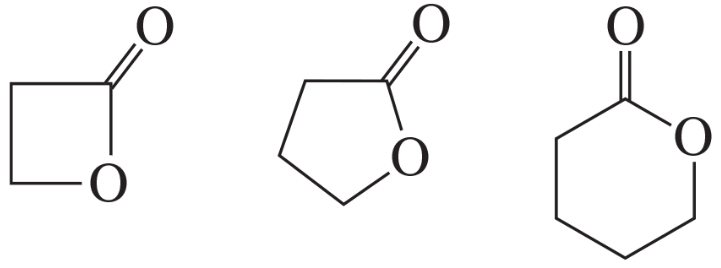
Rank the following compounds from highest wavenumber to lowest wavenumber for their C=O absorption bands:
b.
Problem 26
Which shows an O–H stretch at a larger wavenumber: ethanol dissolved in carbon disulfide or an undiluted sample of ethanol?
Problem 28b
A nitrogen-containing compound shows no absorption band at ~3400 cm-1 and no absorption bands between ~1700 cm-1 and ~1600 cm-1 or between 2260 cm-1 and 2220 cm-1. What class of compound is it?
Problem 29a,b
How can IR spectroscopy be used to distinguish between the following compounds?
a. a ketone and an aldehyde
b. a cyclic ketone and an open-chain ketone
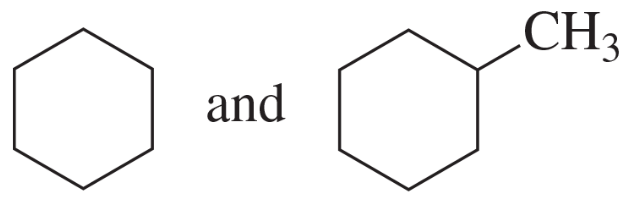
Problem 29e,f
How can IR spectroscopy be used to distinguish between the following compounds?
e. cyclohexene and cyclohexane
f. a primary amine and a tertiary amine
Problem 30c,d
For each of the following pairs of compounds, name one absorption band that can be used to distinguish between them.
c.
d.
Problem 30e,f
For each of the following pairs of compounds, name one absorption band that can be used to distinguish between them.
e.
f.
Problem 31
Which of the following compounds has a vibration that is infrared inactive?
1-butyne, 2-butyne, H2, H2O, Cl2, ethene
Problem 32
Identify the compound that gives the mass spectrum and infrared spectrum shown here.
<IMAGE>
Problem 35
A 4.0 × 10-5 M solution of a compound in hexane shows an absorbance of 0.40 at 252 nm in a cell with a 1 cm light path. What is the molar absorptivity of the compound in hexane at 252 nm?
Problem 36
Predict the λmax of the following compound: