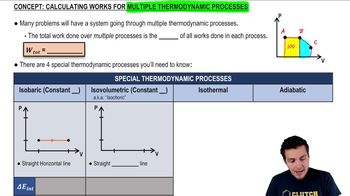
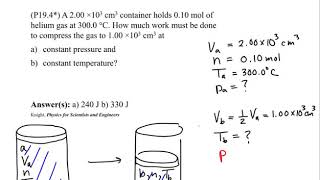
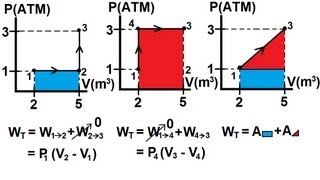
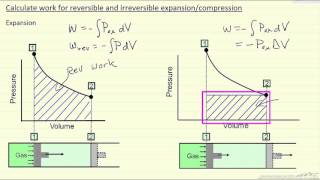
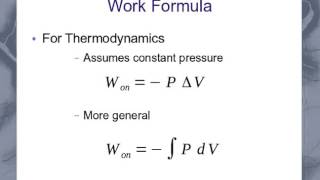
22. The First Law of Thermodynamics
Work Done Through Multiple Processes
22. The First Law of Thermodynamics
Work Done Through Multiple Processes
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
How much work is done on a gas that expands from A to B along the path shown below?
- Multiple Choice
A gas with an initial volume of 0.2 m3 is heated at constant volume, and the pressure increases from 2×105 Pa to 5×105. Then, it compresses at constant pressure until it reaches a final volume of 0.12 m3. Draw the two processes in the PV diagram below and find the total work done by the gas.
- Multiple ChoiceHow much work is done on the gas in the process shown in the figure? Let , , , and .
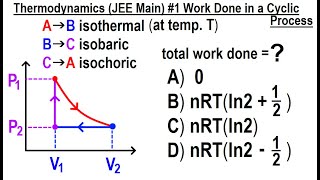
- Open QuestionWork Done in a Cyclic Process. (a) In Fig. 19.7a, consider the closed loop 1 → 3 → 2 → 4 → 1. This is a cyclic process in which the initial and final states are the same. Find the total work done by the system in this cyclic process, and show that it is equal to the area enclosed by the loop.