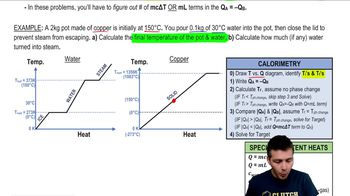
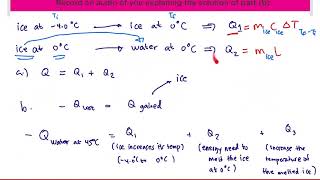
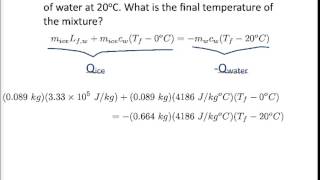
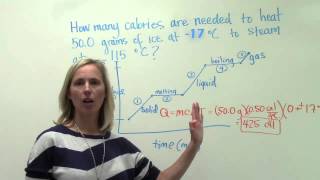20. Heat and Temperature
Advanced Calorimetry: Equilibrium Temperature with Phase Changes
20. Heat and Temperature
Advanced Calorimetry: Equilibrium Temperature with Phase Changes
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Open QuestionA copper pot with a mass of 0.500 kg contains 0.170 kg of water, and both are at 20.0°C. A 0.250-kg block of iron at 85.0°C is dropped into the pot. Find the final temperature of the system, assuming no heat loss to the surroundings.
- Open QuestionA copper calorimeter can with mass 0.100 kg contains 0.160 kg of water and 0.0180 kg of ice in thermal equilibrium at atmospheric pressure. If 0.750 kg of lead at 255°C is dropped into the calorimeter can, what is the final temperature? Assume that no heat is lost to the surroundings.
- Open QuestionA blacksmith cools a 1.20-kg chunk of iron, initially at 650.0°C, by trickling 15.0°C water over it. All of the water boils away, and the iron ends up at 120.0°C. How much water did the blacksmith trickle over the iron?
- Open Question
What will be the final result when equal masses of ice at 0°C and steam at 100°C are mixed together?