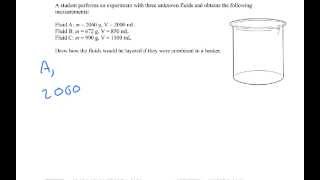
1. Intro to Physics Units
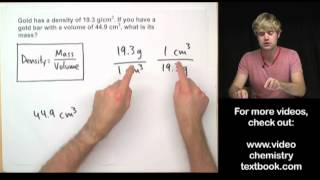
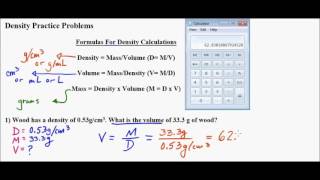
Solving Density Problems
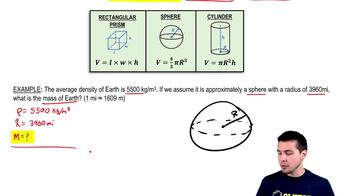
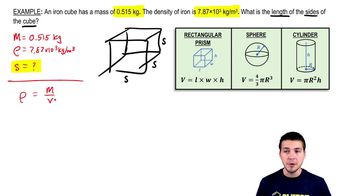
1. Intro to Physics Units
Solving Density Problems
Additional 1 creators.
Learn with other creators
Practice this topic
- Multiple Choice
A wooden cylinder has a radius of 3.5 cm and a height of 6 cm. If the mass is 161 g, what is the density of the wooden cylinder?
1views - Multiple Choice
Copper has a density of 8.96 g/cm3. If a single copper atom as a mass of 1.055×10-25 kg, what is the volume of a copper atom?
1views - Multiple ChoiceYou have three objects in front of you. Object A has a volume of , object B has a volume of , and object C has a volume of . They all have the same mass. What is the correct ranking of the objects from most dense to least dense?
- Open QuestionThe quantity called mass density is the mass per unit volume of a substance. What are the mass densities in basic SI units of the following objects?a. A 215 cm³ solid with a mass of 0.0179 kg.2views
- Open QuestionIn the fall of 2002, scientists at Los Alamos National Laboratory determined that the critical mass of neptunium-237 is about 60 kg. The critical mass of a fissionable material is the minimum amount that must be brought together to start a nuclear chain reaction. Neptunium-237 has a density of 19.5 g/cm3. What would be the radius of a sphere of this material that has a critical mass?2views
- Open QuestionThe nucleus of a uranium atom has a diameter of 1.5×10^−14 m and a mass of 4.0×10^−25 kg . What is the density of the nucleus?2views
- Open Question
A standard baseball has a circumference of approximately 23 cm. If a baseball had the same mass per unit volume (see Tables in Section 1–4) as a neutron or a proton, about what would its mass be?
2views