21. Kinetic Theory of Ideal Gases
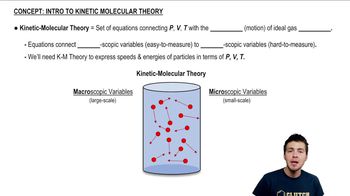
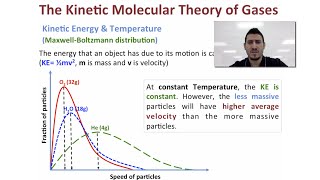
Kinetic-Molecular Theory of Gases
21. Kinetic Theory of Ideal Gases
Kinetic-Molecular Theory of Gases
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Open QuestionA flask contains a mixture of neon (Ne), krypton (Kr), and radon (Rn) gases. Compare (a) the average kinetic energies of the three types of atoms and
- Open Question(a) What is the total translational kinetic energy of the air in an empty room that has dimensions 8.00 m * 12.00 m * 4.00 m if the air is treated as an ideal gas at 1.00 atm?
- Open QuestionConsider a container like that shown in Figure 20.12, with n₁ moles of a monatomic gas on one side and n₂ moles of a diatomic gas on the other. The monatomic gas has initial temperature T₁ᵢ. The diatomic gas has initial temperature T₂ᵢ.b. Show that the equilibrium temperature is
- Open QuestionAt 100℃ the rms speed of nitrogen molecules is 576 m/s. Nitrogen at 100℃ and a pressure of 2.0 atm is held in a container with a 10 cm x 10 cm square wall. Estimate the rate of molecular collisions (collisions/s) on this wall.