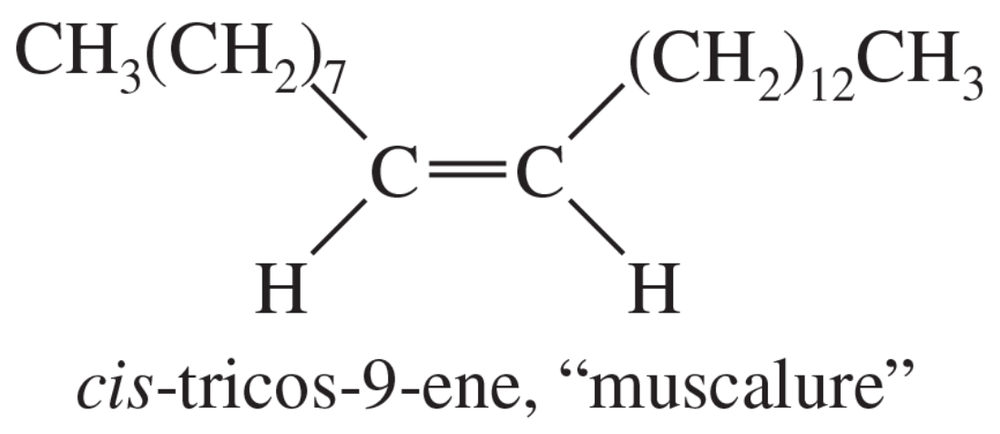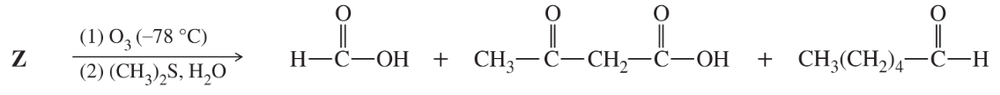Back
BackProblem 31
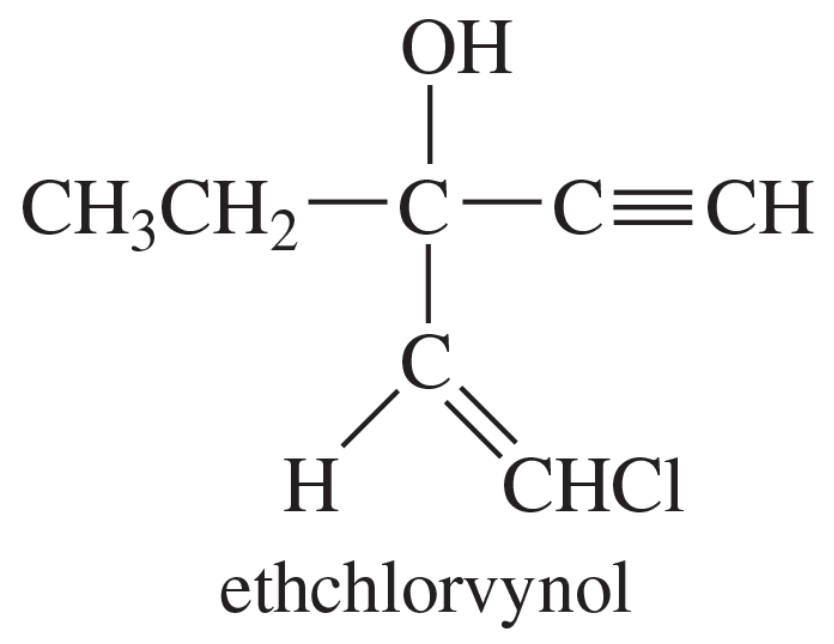
The application box in the margin of states, “The addition of an acetylide ion to a carbonyl group is used in the synthesis of ethchlorvynol, a drug used to cause drowsiness and induce sleep.” Show how you would accomplish this synthesis from acetylene and a carbonyl compound.
Problem 32
Muscalure, the sex attractant of the common housefly, is cis-tricos-9-ene. Most syntheses of alkenes give the more stable trans isomer as the major product. Devise a synthesis of muscalure from acetylene and other compounds of your choice. Your synthesis must give specifically the cis isomer of muscalure.
Problem 33ab,c
Predict the products of reaction of pent-1-yne with the following reagents.
a. 1 equivalent of HCl
b. 2 equivalents of HCl
c. excess H2, Ni
Problem 33d,e,f
Predict the products of reaction of pent-1-yne with the following reagents.
d. H2, Pd/BaSO4, quinoline
e. 1 equivalent of Br2
f. 2 equivalents of Br2
Problem 33g,h,i
Predict the products of reaction of pent-1-yne with the following reagents.
g. cold, dilute KMnO4
h. warm, concd. KMnO4, NaOH
i. Na, liquid ammonia
Problem 33j,k,l
Predict the products of reaction of pent-1-yne with the following reagents.
j. NaNH2
k. H2SO4/HgSO4, H2O
l. Sia2BH, then H2O2, –OH
Problem 34a
Show how you would accomplish the following synthetic transformations. Show all intermediates.
a. 2,2-dibromobutane → but-1-yne
Problem 34b
Show how you would accomplish the following synthetic transformations. Show all intermediates.
b. 2,2-dibromobutane → but-2-yne
Problem 34c
Show how you would accomplish the following synthetic transformations. Show all intermediates.
c. but-1-yne → oct-3-yne
Problem 34d
Show how you would accomplish the following synthetic transformations. Show all intermediates.
d. trans-hex-2-ene → hex-2-yne
Problem 34e
Show how you would accomplish the following synthetic transformations. Show all intermediates.
e. 2,2-dibromohexane → hex-1-yne
Problem 34f
Show how you would accomplish the following synthetic transformations. Show all intermediates.
f. cyclodecyne → cis-cyclodecene
Problem 34g
Show how you would accomplish the following synthetic transformations. Show all intermediates.
g. cyclodecyne → trans-cyclodecene
Problem 34h
Show how you would accomplish the following synthetic transformations. Show all intermediates.
h. hex-1-yne → hexan-2-one, CH3COCH2CH2CH2CH3
Problem 34i
Show how you would accomplish the following synthetic transformations. Show all intermediates.
i. hex-1-yne → hexanal, CH3(CH2)4CHO
Problem 34j
Show how you would accomplish the following synthetic transformations. Show all intermediates.
j. trans-hex-2-ene → cis-hex-2-ene
Problem 35a,b
Show how you would synthesize the following compounds from acetylene and any other needed reagents:
(a) 6-phenylhex-1-en-4-yne
(b) cis-1-phenylpent-2-ene
Problem 36a,b,c
Predict the products formed when CH3CH2–C≡C:–Na+ reacts with the following compounds.
a. ethyl bromide
b. tert-butyl bromide
c. formaldehyde
Problem 36d,e
Predict the products formed when CH3CH2–C≡C:–Na+ reacts with the following compounds.
(d) cyclohexanone
(e) CH3CH2CH2CHO
Problem 37a,b,c
Show how you would synthesize the following compounds, starting with acetylene and any compounds containing no more than four carbon atoms.
a. hex-1-yne
b. hex-2-yne
c. cis-hex-2-ene
Problem 37d,e,f
Show how you would synthesize the following compounds, starting with acetylene and any compounds containing no more than four carbon atoms.
d. trans-hex-2-ene
e. 1,1-dibromohexane
f. 2,2-dibromohexane
Problem 37g,h,i
Show how you would synthesize the following compounds, starting with acetylene and any compounds containing no more than four carbon atoms.
g. pentanal, CH3CH2CH2CH2CHO
h. pentan-2-one, CH3–CO–CH2CH2CH3
i. (±) 3,4-dibromohexane
Problem 38
When treated with hydrogen and a platinum catalyst, an unknown compound X absorbs 5 equivalents of hydrogen to give n-butylcyclohexane. Treatment of X with an excess of ozone, followed by dimethyl sulfide and water, gives the following products:
Propose a structure for the unknown compound X. Is there any uncertainty in your structure?
Problem 39
When compound Z is treated with ozone, followed by dimethyl sulfide and washing with water, the products are formic acid, 3-oxobutanoic acid, and hexanal.
Propose a structure for compound Z. What uncertainty is there in the structure you have proposed?
Problem 40
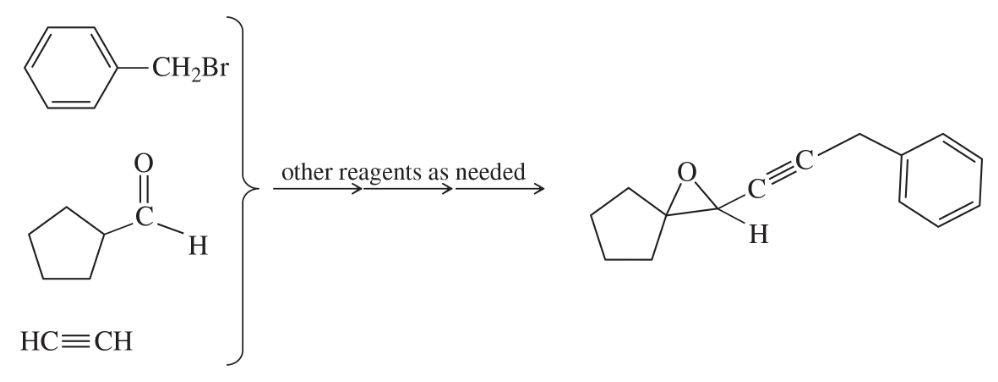
Show how you would convert the following starting materials into the target compound. You may use any additional reagents you need.
Problem 41a,b
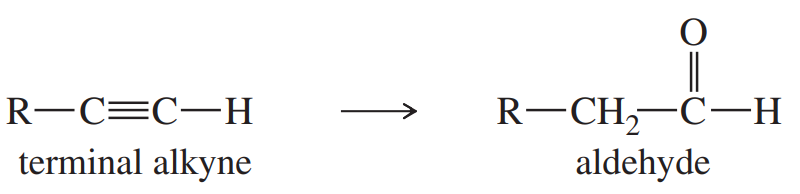
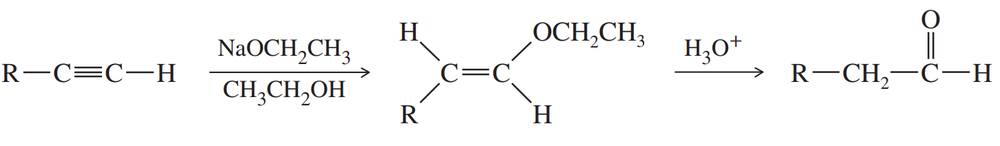
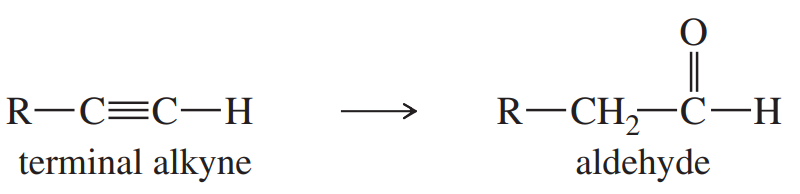
The following functional-group interchange is a useful synthesis of aldehydes.
(a) What reagents were used in this chapter for this transformation? Give an example to illustrate this method.
(b) This functional-group interchange can also be accomplished using the following sequence.
Propose mechanisms for these steps.
Problem 41c
The following functional-group interchange is a useful synthesis of aldehydes.
(c) Explain why a nucleophilic reagent such as ethoxide adds to an alkyne more easily than it adds to an alkene.
Problem 42a
Using any necessary inorganic reagents, show how you would convert acetylene and isobutyl bromide to
(a) meso-2,7-dimethyloctane-4,5-diol, (CH3)2CHCH2CH(OH)CH(OH)CH2CH(CH3)2.
Problem 42b
Using any necessary inorganic reagents, show how you would convert acetylene and isobutyl bromide to
(b) (±)-2,7-dimethyloctane-4,5-diol.
Problem 43a
Deduce the structure of each compound from the information given. All unknowns in this problem have molecular formula C8H12.
(a) Upon catalytic hydrogenation, unknown W gives cyclooctane. Ozonolysis of W, followed by reduction with dimethyl sulfide, gives octanedioic acid, HOOC–(CH2)6–COOH. Draw the structure of W.